Mesentery
| Mesentery | |
|---|---|
|
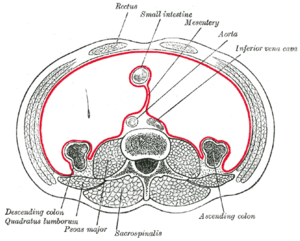
Horizontal disposition of the peritoneum in the lower part of the abdomen. The mesentery is marked with red. | |
|
Vertical disposition of the peritoneum. Main cavity, red; omental bursa, blue. | |
| Details | |
| Identifiers | |
| Latin | Mesenterium |
| MeSH | A01.047.025.600.451 |
| TA | A10.1.02.007 |
| FMA | 7144 |
The mesentery[help 1] is a fold of membranous tissue that arises from the posterior wall of the peritoneal cavity and attaches to the intestinal tract. Within it are the arteries and veins that supply the intestine. The term can be used narrowly to denote just the material that supplies the jejunum and ileum of the small intestine, or broadly to include the right, left and transverse mesocolon, mesoappendix, mesosigmoid and mesorectum.
The human mesentery, also called the mesenteric organ, mainly comprises the small intestinal mesentery, the right, left and transverse mesocolon, mesosigmoid and mesorectum.[1] Conventional teaching has described the mesocolon as a fragmented structure; the small intestinal mesentery, transverse and sigmoid mesocolon all terminate at their insertion into the posterior abdominal wall.[1] Recent advances in gastrointestinal anatomy have demonstrated that the mesenteric organ is actually a single, continuous structure that reaches from the duodenojejunal flexure to the level of the distal mesorectum. This simpler concept has been shown to have significant implications.[1][2]
Structure
Contemporary characterizations of mesenteric anatomy revealed several novel anatomical findings not previously documented. In 2012, the first prospective observational study of the mesocolon was undertaken.[3] 109 patients undergoing open, elective, total abdominal colectomy were studied. Anatomical observations were recorded during the surgery and on the post-operative specimens. These observations included:
- the mesocolon is continuous from ileocaecal to rectosigmoid level;
- a mesenteric confluence occurs at the ileocaecal and rectosigmoid junction as well as at the hepatic and splenic flexures;
- each flexure (and ileocaecal junction) is a complex of peritoneal and omental attachments to the colon centred on a mesenteric confluence;
- the proximal rectum originates at the confluence of the mesorectum and mesosigmoid;
- a plane occupied by perinephric fascia separates the entire apposed small intestinal mesentery and mesocolon from the retroperitoneum. Deep in the pelvis, this fascia coalesces to give rise to presacral fascia.[3]
Flexural anatomy
Flexural anatomy is frequently described as a difficult area. It is simplified when each flexure is considered as being centered on a mesenteric contiguity. The ileocaecal flexure arises at the point where the ileum is continuous with the caecum around the ileocaecal mesenteric flexure. Similarly, the hepatic flexure is formed between the right mesocolon and transverse mesocolon at the mesenteric confluence. The colonic component of the hepatic flexure is draped around this mesenteric confluence. Furthermore, the splenic flexure is formed by the mesenteric confluence between the transverse and left mesocolon. The colonic component of the splenic flexure occurs lateral to the mesenteric confluence. At every flexure, a continuous peritoneal fold lies outside the colonic/mesocolic complex tethering this to the posterior abdominal wall.[1][3]
Segments
The mesoappendix is the portion of the mesentery connecting the ileum to the appendix. It may extend to the tip of the appendix. It encloses the appendicular artery and vein, as well as lymphatic vessels, nerves, and often a lymph node.
Peritoneal folds
Understanding the macroscopic structure of the mesenteric organ meant that associated structures — the peritoneal folds, and congenital and omental adhesions — could be better appraised. The small intestinal mesenteric fold occurs where the small intestinal mesentery folds onto the posterior abdominal wall and continues laterally as the right mesocolon. During mobilization of the small intestinal mesentery from the posterior abdominal wall, this fold is incised, allowing access to the interface between the small intestinal mesentery and the retroperitoneum. The fold continues at the inferolateral boundary of the ileocaecal junction and turns cephalad as the right paracolic peritoneal fold. This fold is divided during lateral to medial mobilization, permitting the surgeon to serially lift the right colon and associated mesentery off the underlying fascia and retroperitoneum. At the hepatic flexure, the right lateral peritoneal fold turns and continues medially as the hepatocolic peritoneal fold. Division of the fold in this location permits separation of the colonic component of the hepatic flexure and mesocolon off the retroperitoneum.[1][3]
Interposed between the hepatic and splenic flexures, the greater omentum adheres to the transverse colon along a further band or fold of peritoneum. Dissection through this allows access to the cephalad (top) surface of the transverse mesocolon. Focal adhesions frequently tether the greater omentum to the cephalad aspect of the transverse mesocolon. The left colon is associated with a similar anatomic configuration of peritoneal folds; the splenic peritoneal fold is contiguous with the left lateral paracolic peritoneal fold at the splenic flexure. Division of the latter similarly allows for the separation of the left colon and associated mesentery off the underlying fascia and frees it from the retroperitoneum. The left lateral paracolic peritoneal fold continues distally at the lateral aspect of the mobile component of the mesosigmoid.[1][3]
Histology
Determination of the macroscopic structure of the mesenteric organ allowed a recent characterisation of the histological and electron microscopic properties.[4] The microscopic structure of the mesocolon and associated fascia is consistent from ileocecal to mesorectal levels. A surface mesothelium and underlying connective tissue is universally apparent. Adipocytes lobules within the body of the mesocolon are separated by fibrous septae arising from submesothelial connective tissue. Where apposed to the retroperitoneum, two mesothelial layers separate the mesocolon and underlying retroperitoneum. Between these is Toldt's fascia, a discrete layer of connective tissue. Lymphatic channels are evident in mesocolic connective tissue and in Toldt’s fascia.[4]
Development
The embryologic forerunner of the gastrointestinal tract is suspended from the posterior abdominal wall by the dorsal mesentery. The gastrointestinal tract and associated dorsal mesentery are subdivided into foregut, midgut and hindgut regions based on the respective blood supply. The foregut is supplied by the celiac trunk, the midgut is supplied by the superior mesenteric artery (SMA) and the hindgut is supplied by the inferior mesenteric artery (IMA). This division is established by the 4th week of intrauterine life. After this, the midgut undergoes a period of rapid elongation, forcing it to herniate through the umbilicus. During herniation, the midgut loop rotates 90o anti-clockwise around the axis of the SMA. The cranial portion of the loop moves to the right and the caudal portion of the loop moves toward the left. This rotation occurs at about the eighth week of development. The cranial portion of the loop will develop into the jejunum, while most of the ileum and the caudal part of the loop eventually form the terminal portion of the ileum, the ascending colon and the initial two thirds of the transverse colon. As the foetus grows larger, the mid-gut loop is drawn back through the umbilicus and undergoes a further 180o rotation, completing a total of 270o rotation. At this point, about 10 weeks, the caecum lies close to the liver. From here it moves in a cranial to caudal direction to eventually lie in the lower right portion of the abdominal cavity. This process brings the ascending colon to lie vertically in the lateral right portion of the abdominal cavity apposed to the posterior abdominal wall. The descending colon occupies a similar position on the left hand side.[5][6]
During these topographic changes, the dorsal mesentery undergoes corresponding changes. Most anatomical and embryological textbooks say that after adopting a final position, the ascending and descending mesocolon disappear during embryogenesis. "Embryology — An Illustrated Colour Text" says, "most of the mid-gut retains the original dorsal mesentery, though parts of the duodenum derived from the mid-gut do not. The mesentery associated with the ascending colon and descending colon is resorbed, bringing these parts of the colon into close contact with the body wall."[6] In "The Developing Human", the author states, "the mesentery of the ascending colon fuses with the parietal peritoneum on this wall and disappears; consequently the ascending colon also becomes retroperitoneal".[7] To reconcile these differences, several theories of embryologic mesenteric development — including the "regression" and "sliding" theories — have been proposed but none have been widely accepted.[6][7]
Clinical significance
Clarifications of the mesenteric anatomy have a clearer understanding of diseases involving the mesentery, examples of which include malrotation and Crohn’s disease (CD). In CD, the mesentery is frequently thickened, rendering haemostasis challenging. In addition, fat wrapping — creeping fat — involves extension of mesenteric fat over the circumference of contiguous gastrointestinal tract, and it has been suggested that this indicates increased mesothelial plasticity. The relationship between mesenteric derangements and mucosal manifestations in CD points to a pathobiologic overlap; some authors say that CD is mainly a mesenteric disorder that secondarily affects the GIT and systemic circulation.[8]
The rationalization of mesenteric and peritoneal fold anatomy permits the surgeon to differentiate both from intraperitoneal adhesions — also called congenital adhesions. These are highly variable among patients and occur in several locations. Congenital adhesions occur between the lateral aspect of the peritoneum overlying the mobile component of the mesosigmoid, and the parietal peritoneum in the left iliac fossa. During lateral to medial approach of mobilizing of the mesosigmoid, these must be divided first before the peritoneum proper can be accessed. Similarly, focal adhesions occur between the undersurface of the greater omentum and the cephalad aspect of the transverse mesocolon. These can be accessed after dividing the peritoneal fold that links the greater omentum and transverse colon. Adhesions here must be divided in order to separate the greater omentum off the transverse mesocolon thus allowing access to the lesser sac proper.[1][2]
Surgery
While the total mesocolic excision (TME) operation has become the surgical gold standard for the management of rectal cancer, this is not so for colon cancer.[1][2][3][9][10][11] Recently, the surgical principles underpinning TME in rectal cancer have been extrapolated to colonic surgery.[12][13][14][15][16][17][18] Total or Complete mesocolic excision (CME), use planar surgery and extensive mesenterectomy (high tie) to minimise breach of the mesentery and maximise lymph nodes yield. Application of this T/CME reduces local five-year recurrence rates in colon cancer from 6.5% to 3.6%, while cancer-related five-year survival rates in patients resected for cure increased from 82.1% to 89.1%.[18]
Radiology
Recent radiologic appraisals of the mesenteric organ have been conducted in the context of the contemporary understanding of mesenteric organ anatomy. When this organ is divided into non-flexural and flexural regions, these can readily be differentiated in most patients on CT imaging. Clarification of the radiological appearance of the human mesentery resonates with the suggestions of Dodd and enables a clearer conceptualization of mesenteric derangements in disease states.[19] This is of immediate relevance in the cancer of spread from colon cancer and perforated diverticular disease, and in pancreatitis where fluid collections in the lesser sac dissect the mesocolon from the retroperitoneum and thereby extend distally within the latter.[20]
History
The classical anatomical description of the mesocolon is credited to British surgeon Sir Frederick Treves in 1885.[21] Treves is known for performing the first appendectomy in England in 1888; he was surgeon to both Queen Victoria and King Edward VII.[22] He studied the human mesentery and peritoneal folds in 100 cadavers and described the right and left mesocolon as vestigial or absent in the human adult. Accordingly, the small intestinal mesentery, transverse and sigmoid mesocolon all terminated or attached at their insertions into the posterior abdominal wall.[21][22] These assertions were included in mainstream surgical, anatomical, embryological and radiologic literature for more than a century.[23][24]
Almost 10 years before Treves, the Austrian anatomist Carl Toldt described the persistence of all portions of the mesocolon into adulthood.[25] Toldt was professor of anatomy in Prague and Vienna; he published his account of the human mesentery in 1879. Toldt identified a fascial plane between the mesocolon and the underlying retroperitoneum formed by the fusion of the visceral peritoneum of the mesocolon with the parietal peritoneum of the retroperitoneum; his later became known as Toldt’s fascia.[25][26]
In 1942, anatomist Edward Congdon also demonstrated that the right and left mesocolon persisted into adulthood and remained separate from the retroperitoneum — extra-retroperitoneal.[27] Radiologist Wylie J. Dodds described this concept in 1986.[19] Dodds extrapolated that unless the mesocolon remained an extra-retroperitoneal structure — separate from the retroperitoneum — only then would the radiologic appearance of the mesentery and peritoneal folds be reconciled with actual anatomy.[19]
Descriptions of the mesocolon by Toldt, Congdon and Dodds have largely been ignored in mainstream literature until recently. A formal appraisal of the mesenteric organ anatomy was conducted in 2012; it echoed the findings of Toldt, Congdon and Dodds.[3] The single greatest advance in this regard was the identification of the mesenteric organ as being contiguous as it spans the gastrointestinal tract from duodenojejunal flexure to mesorectal level.[3]
Lymphangiology
An improved understanding of mesenteric structure and histology has enabled a formal characterization of mesenteric lymphangiology.[4] Stereologic assessments of the lymphatic vessels demonstrate a rich lymphatic network embedded within the mesenteric connective tissue lattice. On average, vessels occur every 0.14 mm (0.0055 in), and within 0.1 mm (0.0039 in) from the mesocolic surfaces — anterior and posterior. Lymphatic channels have also been identified in Toldt’s fascia, though the significance of this is unknown.[4]
Gallery
-

Figure obtained by combining several successive sections of a human embryo of about the fourth week.
-
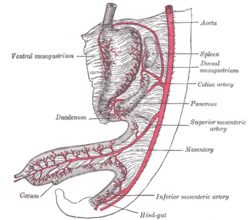
Abdominal part of digestive tube and its attachment to the primitive or common mesentery. Human embryo of six weeks.
-

Mesenteric relation of intestines. Deep dissection. Anterior view.
-
Mesenteric relation of intestines. Deep dissection. Anterior view.
Notes
- ↑ The word mesentery (/ˈmɛzənˌtɛri/) and its New Latin equivalent mesenterium (/ˌmɛzənˈtɛriəm/) use the combining forms mes- + enteron, ultimately from Ancient Greek μεσεντερον (mesenteron), from μέσος (mésos), "middle" + ἔντερον (énteron), "gut", yielding "mid-intestine" or "midgut". The adjectival form is mesenteric (/ˌmɛzənˈtɛrᵻk/.)
References
- 1 2 3 4 5 6 7 8 Coffey JC (August 2013). "Surgical anatomy and anatomic surgery - Clinical and scientific mutualism". The Surgeon 11 (4): 177–82. doi:10.1016/j.surge.2013.03.002. PMID 23597667.
- 1 2 3 Coffey JC, Sehgal R, Culligan K, et al. (June 2014). "Terminology and nomenclature in colonic surgery: universal application of a rule-based approach derived from updates on mesenteric anatomy". Techniques in Coloproctology 18: 789–94. doi:10.1007/s10151-014-1184-2. PMID 24968936.
- 1 2 3 4 5 6 7 8 Culligan K, Coffey JC, Kiran RP, Kalady M, Lavery IC, Remzi FH (April 2012). "The mesocolon: a prospective observational study". Colorectal Disease 14 (4): 421–8; discussion 428–30. doi:10.1111/j.1463-1318.2012.02935.x. PMID 22230129.
- 1 2 3 4 Culligan K, Walsh S, Dunne C, et al. (January 2014). "The Mesocolon: A Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization". Annals of Surgery 260: 1048–56. doi:10.1097/SLA.0000000000000323. PMID 24441808.
- ↑ Ellis, Harold; Mahadevan, Vishy (April 2014). "Anatomy of the caecum, appendix and colon". Surgery 32 (4): 155–8. doi:10.1016/j.mpsur.2014.02.001.
- 1 2 3 Mitchell B, Sharma R. Embryology: An Illustrated Colour Text, 2e. Churchill Livingstone; 2 edition (June 22, 2009). ISBN 978-0702032257.
- 1 2 Moore KL, TPersaud TVN, Torchia MG. The Developing Human: Clinically Oriented Embryology with Student Consult Online Assess, 9th Edition. Saunders; ISBN 978-1437720020
- ↑ Sahebally SM, Burke JP, Chang KH, Kiernan MG, O'Connell PR, Coffey JC (November 2013). "Circulating fibrocytes and Crohn's disease". The British Journal of Surgery 100 (12): 1549–56. doi:10.1002/bjs.9302. PMID 24264775.
- ↑ Sehgal R, Coffey JC (June 2014). "The development of consensus for complete mesocolic excision (CME) should commence with standardisation of anatomy and related terminology". International Journal of Colorectal Disease 29 (6): 763–4. doi:10.1007/s00384-014-1852-8. PMID 24676507.
- ↑ Sehgal R, Coffey JC (July 2014). "Comprehensive standardization of complete mesocolic surgery is now possible". Techniques in Coloproctology 18 (7): 675–6. doi:10.1007/s10151-014-1135-y. PMID 24599764.
- ↑ Sehgal R, Coffey JC (April 2014). "Standardization of the nomenclature based on contemporary mesocolic anatomy is paramount prior to performing a complete mesocolic excision". International Journal of Colorectal Disease 29 (4): 543–4. doi:10.1007/s00384-014-1835-9. PMID 24488141.
- ↑ West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P (September 2008). "Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study". The Lancet Oncology 9 (9): 857–65. doi:10.1016/S1470-2045(08)70181-5. PMID 18667357.
- ↑ Jagoditsch M, Lisborg PH, Jatzko GR, et al. (October 2000). "Long-term prognosis for colon cancer related to consistent radical surgery: multivariate analysis of clinical, surgical, and pathologic variables". World Journal of Surgery 24 (10): 1264–70. doi:10.1007/s002680010252. PMID 11071473.
- ↑ Bokey EL, Chapuis PH, Dent OF, Mander BJ, Bissett IP, Newland RC (July 2003). "Surgical technique and survival in patients having a curative resection for colon cancer". Diseases of the Colon and Rectum 46 (7): 860–6. doi:10.1097/01.DCR.0000074731.78773.BB. PMID 12847357.
- ↑ Søndenaa K, Quirke P, Hohenberger W, et al. (April 2014). "The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery : proceedings of a consensus conference". International Journal of Colorectal Disease 29 (4): 419–28. doi:10.1007/s00384-013-1818-2. PMID 24477788.
- ↑ Storli KE, Søndenaa K, Furnes B, et al. (June 2014). "Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I-II". Techniques in Coloproctology 18 (6): 557–64. doi:10.1007/s10151-013-1100-1. PMID 24357446.
- ↑ Galizia G, Lieto E, De Vita F, et al. (January 2014). "Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study". International Journal of Colorectal Disease 29 (1): 89–97. doi:10.1007/s00384-013-1766-x. PMID 23982425.
- 1 2 Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (May 2009). "Standardized surgery for colonic cancer: complete mesocolic excision and central ligation – technical notes and outcome". Colorectal Disease 11 (4): 354–64; discussion 364–5. doi:10.1111/j.1463-1318.2008.01735.x. PMID 19016817.
- 1 2 3 Dodds WJ, Darweesh RM, Lawson TL, et al. (December 1986). "The retroperitoneal spaces revisited". AJR. American Journal of Roentgenology 147 (6): 1155–61. doi:10.2214/ajr.147.6.1155. PMID 3490750.
- ↑ Koo BC, Chinogureyi A, Shaw AS (February 2010). "Imaging acute pancreatitis". The British Journal of Radiology 83 (986): 104–12. doi:10.1259/bjr/13359269. PMC 3473535. PMID 20139261.
- 1 2 Treves F (March 1885). "Lectures on the Anatomy of the Intestinal Canal and Peritoneum in Man". British Medical Journal 1 (1264): 580–3. doi:10.1136/bmj.1.1264.580. PMC 2255923. PMID 20751205.
- 1 2 Mirilas P, Skandalakis JE (June 2003). "Not just an appendix: Sir Frederick Treves". Archives of Disease in Childhood 88 (6): 549–52. doi:10.1136/adc.88.6.549. PMC 1763108. PMID 12765932.
- ↑ Ellis H. The abdomen and pelvis. In: Ellis H, editor. Clinical anatomy: applied anatomy for students and junior doctors. 12th ed. Blackwell Science; 2010. p. 86.
- ↑ McMinn RH (1994). "The gastrointestinal tract". In McMinn RH. Last's anatomy: regional and applied (9th ed.). London: Langman Group. p. 331e42.
- 1 2 Toldt C (1879). "Bau und wachstumsveranterungen der gekrose des menschlischen darmkanales". Denkschrdmathnaturwissensch 41: 1–56.
- ↑ Toldt C (1919). "Splanchology – general considerations". In Toldt C & Della Rossa A. An atlas of human anatomy for students and physicians 4. New York: Rebman Company. p. 408.
- ↑ Congdon, Edgar D.; Blumberg, Ralph; Henry, William (March 1942). "Fasciae of fusion and elements of the fused enteric mesenteries in the human adult". American Journal of Anatomy 70 (2): 251–79. doi:10.1002/aja.1000700204.
External links
- Anatomy photo:39:01-0100 at the SUNY Downstate Medical Center
- jejunumileum at The Anatomy Lesson by Wesley Norman (Georgetown University)
- McGill
- -1724579760 at GPnotebook
| ||||||||||||||||||||||||||||||||||||||