Mescaline
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
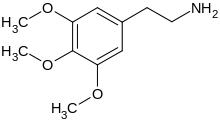
2-(3,4,5-trimethoxyphenyl)ethanamine | |
| Clinical data | |
| AHFS/Drugs.com | entry |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral, intravenous |
| Pharmacokinetic data | |
| Biological half-life | 6 hours |
| Identifiers | |
| CAS Number |
54-04-6 |
| ATC code | None |
| PubChem | CID 4076 |
| ChemSpider |
3934 |
| UNII |
RHO99102VC |
| KEGG |
C06546 |
| ChEBI |
CHEBI:28346 |
| ChEMBL |
CHEMBL26687 |
| Synonyms | 3,4,5-trimethoxyphenethylamine |
| Chemical data | |
| Formula | C11H17NO3 |
| Molar mass | 211.257 g/mol |
| |
| |
| Physical data | |
| Melting point | 35 to 36 °C (95 to 97 °F) |
| Boiling point | 180 °C (356 °F) |
| (verify) | |
Mescaline, or 3,4,5-trimethoxyphenethylamine, is a naturally occurring psychedelic alkaloid of the phenethylamine class, known for its hallucinogenic effects similar to those of LSD and psilocybin.
It occurs naturally in the peyote cactus (Lophophora williamsii),[1] the San Pedro cactus[2] (Echinopsis pachanoi) and in the Peruvian torch (Echinopsis peruviana), and as well in a number of other members of the Cactaceae plant family. It is also found in small amounts in certain members of the Fabaceae (bean) family, including Acacia berlandieri.[3]

History and usage
Peyote has been used for at least 5700 years by Native Americans in Mexico.[5] Europeans noted use of peyote in Native American religious ceremonies upon early contact, notably by the Huichols in Mexico. Other mescaline-containing cacti such as the San Pedro have a long history of use in South America, from Peru to Ecuador.
In traditional peyote preparations, the top of the cactus is cut off, leaving the large tap root along with a ring of green photosynthesizing area to grow new heads. These heads are then dried to make disc-shaped buttons. Buttons are chewed to produce the effects or soaked in water to drink. However, the taste of the cactus is bitter, so contemporary users will often grind it into a powder and pour it in capsules to avoid having to taste it. The usual human dosage is 200–400 milligrams of mescaline sulfate or 178–356 milligrams of mescaline hydrochloride.[6] The average 76 mm (3.0 in) button contains about 25 mg mescaline.[7]
Mescaline was first isolated and identified in 1897 by the German chemist Arthur Heffter[8] and first synthesized in 1919 by Ernst Späth.[9]
In 1955, English politician Christopher Mayhew took part in an experiment for BBC's Panorama, in which he ingested 400 mg of mescaline under the supervision of psychiatrist Humphry Osmond. Though the recording was deemed too controversial and ultimately omitted from the show, Mayhew praised the experience, calling it "the most interesting thing I ever did".[10]
Potential medical usage
Mescaline has a wide array of suggested medical usage, including treatment of alcoholism[11] and depression.[12] However, its status as a Schedule I controlled substance in the Convention on Psychotropic Substances limits availability of the drug to researchers. Because of this, very few studies concerning mescaline's activity and potential therapeutic effects in humans have been conducted since the early 1970s.
Notable users
- Aldous Huxley described his experience with mescaline in the essay The Doors of Perception.
- The sex psychologist Havelock Ellis also tried mescaline.[13][14]
- Hunter S. Thompson wrote an extremely detailed account of his first use of mescaline in First Visit with Mescalito, appearing in his book Songs of the Doomed.
- Psychedelic research pioneer Alexander Shulgin said he was first inspired to explore psychedelic compounds by a mescaline experience.[15]
- Bryan Wynter produced Mars Ascends after trying the substance his first time.[16]
- According to Paul Strathern's book Sartre in 90 Minutes, Jean-Paul Sartre experimented with mescaline, and his description of ultimate reality (in Nausea) as "viscous and obscene" was written under mescaline's influence.
- George Carlin mentions mescaline use during his youth while being interviewed.[17]
- Carlos Santana told in 1989 about his mescaline use in a Rolling Stone interview.[18]
- Michael Cera used real mescaline for the movie Crystal Fairy & the Magical Cactus, as expressed in an interview.[19]
- Melissa Joan Hart: "I experimented with weed, Ecstasy, mushrooms and mescaline for about a year and a half," Hart tells Life and Style magazine.[20]
Biosynthesis of mescaline
Mescaline is biosynthesized from tyrosine or a hydroxylated phenylalanine. In Lophophora williamsii, dopamine converts into mescaline in a biosynthetic pathway involving m-O-methylation and aromatic hydroxylation.[21]

Tyrosine and phenylalanine serve as the metabolic precursors to synthesis of mescaline. Tyrosine can either undergo a decarboxylation via tyrosine decarboxylase to generate tyramine and subsequently undergo an oxidation at carbon 3 by a monophenol hydroxylase or first be hydroxylated by tyrosine hydroxylase to form L-DOPA and decarboxylated by DOPA decarboxylase. These create dopamine, which then experiences methylation by a catechol-O-methyltransferase (COMT) by an S-adenosyl methionine (SAM)-dependent mechanism. The resulting intermediate is then oxidized again by a hydroxylase enzyme, likely monophenol hydroxylase again, at carbon 5, and methylated by COMT. The product, methylated at the two meta positions with respect to the alkyl substituent, experiences a final methylation at the 4 carbon by a guaiacol-O-methyltransferase, which also operates by a SAM-dependent mechanism. This final methylation step results in the production of mescaline.
Phenylalanine serves as a precursor by first being converted to L-tyrosine by L-amino acid hydroxylase. Once converted, it follows the same pathway as described above.[22][23]
Synthetic mescaline
Mescaline was first synthesized in 1919 by Ernst Späth from 3,4,5-trimethoxybenzoyl chloride.[24] Subsequent to this, numerous approaches utilizing different starting materials have been developed. Notable examples include the following:
- Hofmann rearrangement of 3,4,5-trimethoxyphenylpropionamide.[25]
- Cyanohydrin reaction between potassium cyanide and 3,4,5-Trimethoxybenzaldehyde followed by acetylation and reduction.[26][27]
- Henry reaction of 3,4,5-Trimethoxybenzaldehyde with nitromethane followed by nitro compound reduction of ω-nitrotrimethoxystyrene.[28][29][30][31][32][33][34]
- Ozonolysis of elemicin followed by reductive amination.[35]
- Ester reduction of Eudesmic acid's methyl ester followed by halogenation, Kolbe nitrile synthesis, and nitrile reduction.[36][37][38]
- Amide reduction of 3,4,5-trimethoxyphenylacetamide.[39]
Pharmacokinetics
Tolerance builds with repeated usage, lasting for a few days. Mescaline causes cross-tolerance with other serotonergic psychedelics such as LSD and psilocybin.[40]
About half the initial dosage is excreted after 6 hours, but some studies suggest that it is not metabolized at all before excretion. Mescaline appears to not be subject to metabolism by CYP2D6[41] and between 20% and 50% of mescaline is excreted in the urine unchanged, and the rest being excreted as the carboxylic acid form of mescaline, a likely result of MAO degradation.[42] The LD50 of mescaline has been measured in various animals: 212 mg/kg i.p. (mice), 132 mg/kg i.p. (rats), and 328 mg/kg i.p. (guinea pigs).
Behavioral and non-behavioral effects
Mescaline induces a psychedelic state similar to those produced by LSD and psilocybin, but with unique characteristics. Subjective effects may include altered thinking processes, an altered sense of time and self-awareness, and closed- and open-eye visual phenomena.
Prominence of color is distinctive, appearing brilliant and intense. Recurring visual patterns observed during the mescaline experience include stripes, checkerboards, angular spikes, multicolor dots, and very simple fractals that turn very complex. Aldous Huxley described these self-transforming amorphous shapes as like animated stained glass illuminated from light coming through the eyelids. Like LSD, mescaline induces distortions of form and kaleidoscopic experiences but they manifest more clearly with eyes closed and under low lighting conditions.
As with LSD, synesthesia can occur especially with the help of music.[43] An unusual but unique characteristic of mescaline use is the "geometricization" of three-dimensional objects. The object can appear flattened and distorted, similar to the presentation of a Cubist painting.[44]
Mescaline elicits a pattern of sympathetic arousal, with the peripheral nervous system being a major target for this substance.[43] Effects typically begin a while after ingestion, and may last a long time depending on dosage.
Mechanism of action
Mescaline is produced when products of natural mammalian catecholamine-based neuronal signalling such as dopamine and serotonin are subjected to additional metabolism via methylation, and mescaline's hallucinogenic properties stem from its structural similarities with these two neurotransmitters. In plants, this compound may be the end-product of a pathway utilizing catecholamines as a method of stress response, similar to how animals may release compounds such as cortisol when stressed. The in vivo function of catecholamines has not been investigated, but they may function as antioxidants, as developmental signals, and as integral cell wall components that resist degradation from pathogens. The deactivation of catecholamines via methylation produces alkaloids such as mescaline.[22]
Mescaline acts similarly to other psychedelic agents.[45] It binds to and activates the serotonin 5-HT2A receptor with a high affinity.[46] How activating the 5-HT2A receptor leads to psychedelia is still unknown, but it likely somehow involves excitation of neurons in the prefrontal cortex.[47] Mescaline is also known to bind to and activate the serotonin 5-HT2C receptor.[48]
Difluoromescaline and trifluoromescaline are more potent than mescaline, as is its amphetamine homologue trimethoxyamphetamine.[49][50]
Legality
United States
In the United States, mescaline was made illegal in 1970 by the Comprehensive Drug Abuse Prevention and Control Act.[51] The drug was prohibited internationally by the 1971 Convention on Psychotropic Substances[52] and is categorized as a Schedule I hallucinogen by the CSA. Mescaline is legal only for certain religious groups (such as the Native American Church) and in scientific and medical research. In 1990, the Supreme Court ruled that the state of Oregon could bar the use of mescaline in Native American religious ceremonies. The Religious Freedom Restoration Act (RFRA) in 1993 allowed the use of peyote in religious ceremony but, in 1997, the Supreme Court ruled that the RFRA is unconstitutional when applied against states. However, in a subsequent case, the Court held that the government had not carried the burden under the Religious Freedom Restoration Act of showing a compelling interest that allowed no exception to the ban on the use of the drug to accommodate a sincere religious practice.[53] Thus, the current state of the law is that, while the federal government may not restrict use of peyote in ceremony, individual states do have a right to restrict its use.[54] Many states, including the state of Utah, have legalized peyote usage with "sincere religious intent", or within a religious organization, regardless of race.[55]
While mescaline-containing cacti of the genus Echinopsis are technically controlled substances under the Controlled Substances Act, they are commonly sold publicly as ornamental plants.
United Kingdom
In the United Kingdom, mescaline in purified powder form is a Class A drug. However, dried cactus can be bought and sold legally.[56]
Other countries
Mescaline is considered a schedule 9 substance in Australia under the Poisons Standard (October 2015).[57] A schedule 9 substance is classified as "Substances with a high potential for causing harm at low exposure and which require special precautions during manufacture, handling or use. These poisons should be available only to specialised or authorised users who have the skills necessary to handle them safely. Special regulations restricting their availability, possession, storage or use may apply." [57]
The peyote cacti and other mescaline-containing plants such as San Pedro are illegal in Western Australia, Queensland, and the Northern Territory, whilst in other states such as Tasmania, Victoria and New South Wales, they are legal for ornamental and gardening purposes.
In Canada, France, The Netherlands and Germany, mescaline in raw form and dried mescaline-containing cacti are considered an illegal drug. However, anyone may grow and use peyote, or Lophophora williamsii, as well as Echinopsis Pachanoi and Echinopsis Peruviana without restriction, as it is specifically exempt from legislation.[1] In Canada, mescaline is classified as a schedule III drug under the Controlled Drugs and Substances Act, whereas peyote is exempt.[58]
See also
- List of psychedelic plants
- Psychedelic experience
- Psychoactive drug
- Entheogen
- The Doors of Perception
- Mind at Large (concept in The Doors of Perception)
- The Psychedelic Experience: A Manual Based on the Tibetan Book of the Dead
References
- 1 2 Drug Identification Bible. Grand Junction, CO: Amera-Chem, Inc. 2007. ISBN 0-9635626-9-X.
- ↑ Crosby, D.M.; and McLaughlin, J.L. (December 1973). "Cactus Alkaloids. XIX Crystallization of Mescaline HCl and 3-Methoxytyramine HCl from Trichocereus panchanoi" (PDF). Lloydia and the Journal of Natural Products 36 (4): 416–418. PMID 4773270. Retrieved 13 December 2013.
- ↑ T.D.A. Forbes, B.A. Clement. "Chemistry of Acacia's from South Texas" (PDF). Texas A&M Agricultural Research & Extension Center at Uvalde. Archived from the original (PDF) on 2012
- ↑ http://mescaline.com/exp/
- ↑ El-Seedi HR, De Smet PA, Beck O, Possnert G, Bruhn JG (October 2005). "Prehistoric peyote use: alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas". J Ethnopharmacol 101 (1–3): 238–42. doi:10.1016/j.jep.2005.04.022. PMID 15990261.
- ↑ "#96 M – Mescaline (3,4,5-Trimethoxyphenethylamine)". PIHKAL. Erowid.org. Retrieved 7 September 2011
- ↑ AJ Giannini, AE Slaby, MC Giannini (1982). Handbook of Overdose and Detoxification Emergencies. New Hyde Park, NY.: Medical Examination Publishing Company. ISBN 978-0-87488-182-0
- ↑ "Arthur Heffter". Character Vaults. Erowid.org. Retrieved 9 January 2013
- ↑ Manske & Holmes (1953). "The Alkaloids". Academic Press (Erowid.org) 3: 324–8
|chapter=ignored (help) - ↑ "Panorama: The Mescaline Experiment". February 2005. Archived from the original on 26 July 2012
- ↑ "Could LSD treat alcoholism?". abcnews.go.com. 9 March 2012. Retrieved 5 October 2012
- ↑ "Magic Mushrooms could treat depression". news.discovery.com. 23 January 2012. Retrieved 9 January 2013
- ↑ Ellis, Havelock. "Mescal: A New Artificial Paradise," The Contemporary Review, Vol. LXXIII, 1898.
- ↑ A James Giannini (1997). Drugs of Abuse (Second ed.). Los Angeles: Practice Management Information Corp. ISBN 1-57066-053-0.
- ↑ "Alexander Shulgin: why I discover psychedelic substances". Luc Sala interview. Mexico. 1996
- ↑ Bird, Michael. "100 Ideas that Changed Art." London: Laurence King Publishing, 2012. Print.
- ↑ Jay Dixit (23 June 2008). "George Carlin's Last Interview". Psychology Today.
- ↑ Rolling Stone 1989, Santana at Woodstock
- ↑ Boardman, Madeline (10 July 2013). "Michael Cera Took Drugs On-Camera". Huffington Post.
- ↑ "Melissa Joan Hart details shocking drug use in new tell-all". Fox News. 26 September 2013.
- ↑ Paul M. Dewick (2009). Medicinal Natural Products: A Biosynthetic Approach. United Kingdom: John Wiley & Sons. pp. 335–336. ISBN 978-0-471-49641-0
- 1 2 Kulma; Szopa, Jan; et al. (March 2007). "Catecholamies are active compounds in plants". Plant Science 172 (3): 433–440. doi:10.1016/j.plantsci.2006.10.013.
- ↑ Rosengarten; Friedhoff, AJ; et al. (1976). "A review of recent studies of the biosynthesis and excretion of hallucinogens formed by methylation of neurotransmitters or related substances". Schizophrenia Bulletin 2 (1): 90–105. doi:10.1093/schbul/2.1.90. PMID 779022.
- ↑ Späth, Ernst (1919). "Über dieAnhalonium-Alkaloide". Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 40 (2): 129–154. doi:10.1007/BF01524590.
- ↑ K. H. Slotta; and H. Heller (1930). "Über β-Phenyl-äthylamine, I. Mitteil.: Mezcalin und mezcalin-ähnliche Substanzen". Berichte der deutschen chemischen Gesellschaft (A and B Series) 63 (11): 3029–3044. doi:10.1002/cber.19300631117.
- ↑ Amos, D. (1964). "Preparation of Mescaline from Eucalypt Lignin". Australian Journal of Pharmacy 49: 529.
- ↑ Kindler, Karl; and Peschke, Wilhelm (1932). "Über neue und über verbesserte Wege zum Aufbau von pharmakologisch wichtigen Aminen VI. Über Synthesen des Meskalins". Archiv der Pharmazie 270 (7): 410–413. doi:10.1002/ardp.19322700709.
- ↑ Benington, Fred; and Morin, Richard (1951). "An Improved Synthesis of Mescaline". Journal of the American Chemical Society 73 (3): 1353–1353. doi:10.1021/ja01147a505.
- ↑ Shulgin, Alexander; and Shulgin, Ann (1991). PiHKAL: A Chemical Love Story. Lafayette, CA: Transform Press. p. 703. ISBN 9780963009609.
- ↑ Hahn, Georg; and Rumpf, Fritz (1938). "Über β-[Oxy-phenyl]-äthylamine und ihre Umwandlungen, V. Mitteil.: Kondensation von Oxyphenyl-äthylaminen mit α-Ketonsäuren". Berichte der deutschen chemischen Gesellschaft (A and B Series) 71 (10): 2141–2153. doi:10.1002/cber.19380711022.
- ↑ Toshitaka, Ohshita; Hiroaka, Ando (1992). "Synthesis of Phenethylamine Derivatives as Hallucinogen" (PDF). Japanese Journal of Toxicology and Environmental Health 38 (6): 571–580. Retrieved 20 June 2014.
- ↑ Ramirez, F.; Erne, Max (1950). "Über die Reduktion von β-Nitrostyrolen mit Lithiumaluminiumhydrid". Helvetica Chimica Acta 33 (4): 912–916. doi:10.1002/hlca.19500330420.
- ↑ Szyszka, G.; Slotta, K. H. (1933). "Über β-Phenyl-äthylamine.III. Mitteilung: Neue Darstellung von Mescalin". Journal für Praktische Chemie 137 (9-12): 339–350. doi:10.1002/prac.19331370907.
- ↑ Burger, Alfred; Ramirez, Fausto A. (1950). "The Reduction of Phenolic β-Nitrostyrenes by Lithium Aluminum Hydride". Journal of the American Chemical Society 72 (6): 2781–2782. doi:10.1021/ja01162a521.
- ↑ Hahn, Georg; and Wassmuth, Heinrich (1934). "Über β-[Oxyphenyl]-äthylamine und ihre Umwandlungen, I. Mitteil.: Synthese des Mezcalins". Berichte der deutschen chemischen Gesellschaft (A and B Series) 67 (4): 696–708. doi:10.1002/cber.19340670430.
- ↑ Makepeace, Tsao (1951). "A New Synthesis of Mescaline". Journal of the American Chemical Society 71 (11): 5495–5496. doi:10.1021/ja01155a562.
- ↑ Dornow, Alfred; and Petsch, Günther (1952). "Über die Darstellung des Oxymezcalins und Mezcalins 2. Mitteilung". Archiv der Pharmazie 285 (7): 323–326. doi:10.1002/ardp.19522850704.
- ↑ Ikan, Raphael (1991). Natural Products: A Laboratory Guide 2nd Ed. San Diego: Academic Press, Inc. pp. 232–235. ISBN 0123705517.
- ↑ Banholzer, K.; Campbell, Tod W.; Schmid, H. (1952). "Notiz über eine neue Synthese von Mezcalin, N-Methyl- und N-Dimethylmezcalin". Helvetica Chimica Acta 35 (5): 1577–1581. doi:10.1002/hlca.19520350519.
- ↑ Michael Valentine Smith. "Psychedelics and Society". Erowid.org. Retrieved 6 April 2012
- ↑ Wu D, Otton SV, Inaba T, Kalow W, Sellers EM (June 1997). "Interactions of amphetamine analogs with human liver CYP2D6". Biochem. Pharmacol. 53 (11): 1605–12. doi:10.1016/S0006-2952(97)00014-2. PMID 9264312.
- ↑ Cochin J, Woods LA, Seevers MH (February 1951). "The absorption, distribution and urinary excretion of mescaline in the dog". J. Pharmacol. Exp. Ther. 101 (2): 205–9. PMID 14814616.
- 1 2 Diaz, Jaime (1996). How Drugs Influence Behavior. Englewood Cliffs: Prentice Hall. ISBN 978-0-02-328764-0
- ↑ A. James Giannini, Andrew E. Slaby (1989). Drugs of Abuse. Oradell, NJ.: Medical Economics Books. pp. 207–239. ISBN 978-0-87489-499-8
- ↑ Nichols DE (February 2004). "Hallucinogens". Pharmacol. Ther. 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ↑ Monte AP, Waldman SR, Marona-Lewicka D, et al. (September 1997). "Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives". J. Med. Chem. 40 (19): 2997–3008. doi:10.1021/jm970219x. PMID 9301661.
- ↑ Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R (June 2007). "Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex". Proc. Natl. Acad. Sci. U.S.A. 104 (23): 9870–5. doi:10.1073/pnas.0700436104. PMC 1887564. PMID 17535909.
- ↑ "Neuropharmacology of Hallucinogens". Erowid.org. 27 March 2009. Retrieved 7 September 2011
- ↑ Trachsel, D. (2012). "Fluorine in psychedelic phenethylamines". Drug Testing and Analysis 4 (7–8): 577–590. doi:10.1002/dta.413. PMID 22374819.
- ↑ Shulgin, Alexander. "#157 TMA - 3,4,5-TRIMETHOXYAMPHETAMINE". PiHKAL: A Chemical Love Story. Erowid.org. Retrieved 9 January 2013
- ↑ United States Department of Justice. "Drug Scheduling". Retrieved 2 November 2007.
- ↑ "List of psychotropic substances under international control" (PDF). International Narcotics Control Board. Retrieved 27 January 2008.
- ↑ "Gonzales, Attorney General, Et Al. V. O Centro Espirita Beneficente Uniao Do Vegetal Et Al". Caselaw.lp.findlaw.com. Retrieved 7 September 2011.
- ↑ "Uses Drugs of Abuse—Origins and Effects – Hallucinogens". libraryindex.com. Retrieved 6 April 2012.
- ↑ "State v. Mooney". utcourts.gov. Retrieved 5 October 2012.
- ↑ "2007 U.K. Trichocereus Cacti Legal Case Regina v. Saul Sette". Erowid.org. June 2007. Retrieved 6 April 2012
- 1 2 Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
- ↑ "Justice Laws Search". laws-lois.justice.gc.ca. Retrieved 5 October 2012.
External links
| Wikimedia Commons has media related to Mescaline. |
- National Institutes of Health – National Institute on Drug Abuse Hallucinogen InfoFacts
- Mescaline at Erowid
- PiHKAL entry
- Mescaline entry in PiHKAL • info
- Film of Christopher Mayhew's mescaline experiment on YouTube
- Mescaline: The Chemistry and Pharmacology of its Analogs, an essay by Alexander Shulgin
- Mescaline on the Mexican Border
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||