Mercury cycle
The mercury cycle is a biogeochemical cycle involving mercury. Mercury is notable for being the only metal which is liquid at room temperature. It is a volatile metal and evaporates, though it takes quite a while to do so.
Processes
Most natural mercury occurs as cinnabar,[1] HgS. Here mercury (Hg2+) is bound very tightly to sulfur, but weathering slowly releases the mercury to the environment. There are also trace amounts of mercury in coal. Mining mercury or burning coal results in releasing mercury. Volcanoes and forest fires are also sources of mercury.
Chlorine factories, among other sources, release mercury into the atmosphere.[2] This mercury is deposited back onto land and water. Inorganic mercury can be converted by bacteria into the organometallic cation known as methylmercury, CH3Hg+, which bioaccumulates in fish such as tuna and swordfish.[2] Over long periods of time, some mercury recombines with sulfur and is buried in sediments. Then, the cycle repeats itself.[2]
Releases of mercury in the environment
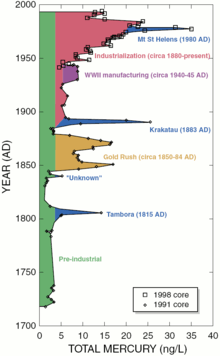
Natural sources
Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.[3]
Natural sources, such as volcanoes, are responsible for approximately half of atmospheric mercury emissions.
Anthropogenic emissions of mercury
The human-generated half can be divided into the following estimated percentages:[4][5][6]
- 65% from stationary combustion, of which coal-fired power plants are the largest aggregate source (40% of U.S. mercury emissions in 1999). This includes power plants fueled with gas where the mercury has not been removed. Emissions from coal combustion are between one and two orders of magnitude higher than emissions from oil combustion, depending on the country.[4]
- 11% from gold production. The three largest point sources for mercury emissions in the U.S. are the three largest gold mines. Hydrogeochemical release of mercury from gold-mine tailings has been accounted as a significant source of atmospheric mercury in eastern Canada.[7]
- 6.8% from non-ferrous metal production, typically smelters.
- 6.4% from cement production.
- 3.0% from waste disposal, including municipal and hazardous waste, crematoria, and sewage sludge incineration.
- 3.0% from caustic soda production.
- 1.4% from pig iron and steel production.
- 1.1% from mercury production, mainly for batteries.
- 2.0% from other sources.
The above percentages are estimates of the global human-caused mercury emissions in 2000, excluding biomass burning, an important source in some regions.[4]
Current atmospheric mercury contamination in outdoor urban air is (0.01–0.02 µg/m3) indoor concentrations are significantly elevated over outdoor concentrations, in the range 0.0065–0.523 µg/m3 (average 0.069 µg/m3).[8]
The magnitude of Hg emissions to the atmosphere from Chinese anthropogenic sources has been estimated to be in the range of 500-700 tons per year, comprising a significant proportion of the global total anthropogenic emissions.[9]
Mercury also enters into the environment through the improper disposal (e.g., land filling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats.[10] Due to health concerns (see below), toxics use reduction efforts are cutting back or eliminating mercury in such products. For example, most thermometers now use pigmented alcohol instead of mercury. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are being replaced by electronic thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
The United States Clean Air Act, passed in 1990, put mercury on a list of toxic pollutants that need to be controlled to the greatest possible extent. Thus, industries that release high concentrations of mercury into the environment agreed to install maximum achievable control technologies (MACT). In March 2005 EPA rule[11] added power plants to the list of sources that should be controlled and a national cap and trade rule was issued. States were given until November 2006 to impose stricter controls, and several States are doing so. The rule was being subjected to legal challenges from several States in 2005 and decision was made in 2008. The Clean Air Mercury Rule was struck down by a Federal Appeals Court on February 8, 2008. The rule was deemed insufficient to protect the health of persons living near coal-fired power plants. The court opinion cited the negative impact on human health from coal-fired power plants' mercury emissions documented in the EPA Study Report to Congress of 1998.[12]
Historically, one of the largest releases was from the Colex plant, a lithium-isotope separation plant at Oak Ridge. The plant operated in the 1950s and 1960s. Records are incomplete and unclear, but government commissions have estimated that some two million pounds of mercury are unaccounted for.[13]
One of the worst industrial disasters in history was caused by the dumping of mercury compounds into Minamata Bay, Japan. The Chisso Corporation, a fertilizer and later petrochemical company, was found responsible for polluting the bay from 1932 to 1968. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease.[14]
Toxicity of mercury
Mercury and most of its compounds are extremely toxic and are generally handled with care; in cases of spills involving mercury (such as from certain thermometers or fluorescent light bulbs) specific cleaning procedures are used to avoid toxic exposure.[15] Essentially, it is recommended to physically merge smaller droplets on hard surfaces, combining them into a single larger pool for easier removal by using an eyedropper, or by pushing it into a disposable container which must then be dealt with according to local regulations. Vacuum cleaners and brooms should not be used because they cause greater dispersal of the mercury. Afterwards, sulfur powder, zinc powder, or some other element that readily forms an amalgam (alloy) with mercury (e.g. finely divided Cu or Bi) at ordinary temperatures is sprinkled over the area and subsequently collected and properly disposed of. Cleaning porous surfaces and clothing is not effective at removing all traces of mercury and it is therefore advised to discard these kinds of items should they be exposed to a mercury spill.
Mercury can be inhaled and absorbed through the skin and mucous membranes, so containers of mercury are securely sealed to avoid spills and evaporation. Heating of mercury, or compounds of mercury that may decompose when heated, is always carried out with adequate ventilation in order to avoid exposure to mercury vapor. The most toxic forms of mercury are its organic compounds, such as dimethylmercury and methylmercury. However, inorganic compounds, such as cinnabar are also highly toxic by ingestion or inhalation of the dust.[16] Mercury can cause both chronic and acute poisoning.
Occupational exposure
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The World Health Organization, OSHA, and NIOSH all treat mercury as an occupational hazard, and have established specific occupational exposure limits. Environmental releases and disposal of mercury are regulated in the U.S. primarily by the United States Environmental Protection Agency.
Case control studies have shown effects such as tremors, impaired cognitive skills, and sleep disturbance in workers with chronic exposure to mercury vapor even at low concentrations in the range 0.7–42 μg/m3.[17][18] A study has shown that acute exposure (4 – 8 hours) to calculated elemental mercury levels of 1.1 to 44 mg/m3 resulted in chest pain, dyspnea, cough, hemoptysis, impairment of pulmonary function, and evidence of interstitial pneumonitis.[19] Acute exposure to mercury vapor has been shown to result in profound central nervous system effects, including psychotic reactions characterized by delirium, hallucinations, and suicidal tendency. Occupational exposure has resulted in broad-ranging functional disturbance, including erethism, irritability, excitability, excessive shyness, and insomnia. With continuing exposure, a fine tremor develops and may escalate to violent muscular spasms. Tremor initially involves the hands and later spreads to the eyelids, lips, and tongue. Long-term, low-level exposure has been associated with more subtle symptoms of erethism, including fatigue, irritability, loss of memory, vivid dreams, and depression.[20][21]
Treatment
Research on the treatment of mercury poisoning is limited. Currently available drugs for acute mercurial poisoning include chelators N-acetyl-D, L-penicillamine (NAP), British Anti-Lewisite (BAL), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and dimercaptosuccinic acid (DMSA). In one small study including 11 construction workers exposed to elemental mercury, patients were treated with DMSA and NAP.[22] Chelation therapy with both drugs resulted in the mobilization of a small fraction of the total estimated body mercury. DMSA was able to increase the excretion of mercury to a greater extent than NAP.[23]
Fish
Fish and shellfish have a natural tendency to concentrate mercury in their bodies, often in the form of methylmercury, a highly toxic organic compound of mercury. Species of fish that are high on the food chain, such as shark, swordfish, king mackerel, albacore tuna, and tilefish contain higher concentrations of mercury than others. As mercury and methylmercury are fat soluble, they primarily accumulate in the viscera, although they are also found throughout the muscle tissue.[24] When this fish is consumed by a predator, the mercury level is accumulated. Since fish are less efficient at depurating than accumulating methylmercury, fish-tissue concentrations increase over time. Thus species that are high on the food chain amass body burdens of mercury that can be ten times higher than the species they consume. This process is called biomagnification. Mercury poisoning happened this way in Minamata, Japan, now called Minamata disease.
Regulations
Use of mercury in products
European Union
In the European Union, the directive on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (see RoHS) bans mercury from certain electrical and electronic products, and limits the amount of mercury in other products to less than 1000 ppm.[25] There are restrictions for mercury concentration in packaging (the limit is 100 ppm for sum of mercury, lead, hexavalent chromium and cadmium) and batteries (the limit is 5 ppm).[26] In July 2007, the European Union also banned mercury in non-electrical measuring devices, such as thermometers and barometers. The ban applies to new devices only, and contains exemptions for the health care sector and a two-year grace period for manufacturers of barometers. [27]
Norway
Norway enacted a total ban on the use of mercury in the manufacturing and import/export of mercury products, effective January 1, 2008.[28] In 2002, several lakes in Norway were found to have a poor state of mercury pollution, with an excess of 1 mg/g of mercury in their sediment.[29]
United States
In the United States, the Environmental Protection Agency is charged with regulating and managing mercury contamination. Several federal laws give the EPA this authority, including the Clean Air Act, the Clean Water Act, the Resource Conservation and Recovery Act, and the Safe Drinking Water Act. Additionally, the Mercury-Containing and Rechargeable Battery Management Act, passed in 1996, phases out the use of mercury in batteries, and provides for the efficient and cost-effective disposal of many types of used batteries.[30] North America contributed approximately 11% of the total global anthropogenic mercury emissions in 1995.[31]
Regulation of mercury air pollution
International Law
Minamata Convention
On 19 January 2013, negotiations on a global legally binding instrument on mercury that had commenced in 2009 concluded with 147 governments agreeing to the draft convention text for the Minamata Convention on Mercury. The draft Minamata Convention on Mercury, on which there will be no further negotiations, is scheduled to be adopted and opened for signature at a Conference of Plenipotentiaries (Diplomatic Conference) in Kumamoto and Minamata, Japan from 10 to 11 October 2013. The formal objective of the Convention is to protect human health and the environment from anthropogenic emissions and releases of mercury and mercury compounds (Article 1). The Parties agreed in Article 8 to control and “where feasible” reduce emissions of mercury and mercury compounds, (i.e. “total mercury”) to the atmosphere through measures to control emissions from point source categories such as coal-fired power stations and non-ferrous metal smelters (e.g. aluminium smelters).
United States
In the United States, the Environmental Protection Agency is charged with regulating and managing mercury contamination. Several federal laws give the EPA this authority, including the Clean Air Act.
The United States Clean Air Act, passed in 1990, put mercury on a list of toxic pollutants that need to be controlled to the greatest possible extent. Thus, industries that release high concentrations of mercury into the environment agreed to install maximum achievable control technologies (MACT).
On 15 March 2005 the USEPA issued the Clean Air Mercury Rule. The intent of this rule is to cap and reduce mercury emissions from coal-fired power plants.
In March 2005 EPA rule[11] added power plants to the list of sources that should be controlled and a national cap and trade rule was issued. States were given until November 2006 to impose stricter controls, and several States are doing so.
The rule was being subjected to legal challenges from several States in 2005 and a decision was made in 2008. The Clean Air Mercury Rule was struck down by a Federal Appeals Court on February 8, 2008. The rule was deemed not sufficient to protect the health of persons living near coal-fired power plants. The court opinion cited the negative impact on human health from coal-fired power plants' mercury emissions documented in the EPA Study Report to Congress of 1998.[12]
EU
On April 4, 2001, the European Council approved the Protocol on Heavy Metals in order to reduce emissions of heavy metals that are subject to long-range transboundary atmospheric transport and are likely to have adverse effects on human health and the environment. The 2001 EU Large Combustion Plant Directive considers stationary combustion plants as one of the main sources of emission of NOx, SOx, particulates and heavy metals into the atmosphere. With the application of the European Directives 96/61/CE (Integrated pollution prevention and control) and 96/62/CE (Ambient air quality assessment and management) in which emission limits for toxic trace elements, such as mercury, are clearly established, energy production plants using coal must control mercury emissions. In January 2005, the European Commission adopted a mercury strategy for reducing mercury levels in the environment.
See also
References
- ↑ Rytuba, James J. (August 2, 2002). "Mercury from mineral deposits and potential environmental impact". Environmental Geology (Springer Science+Business Media) 43 (3): 326–338. doi:10.1007/s00254-002-0629-5. Retrieved March 20, 2012.
- 1 2 3 "Mercury: Overview". Oceana. Oceana: Protecting the World's Oceans. 2012. Retrieved February 28, 2012.
- ↑ "Glacial Ice Cores Reveal A Record of Natural and Anthropogenic Atmospheric Mercury Deposition for the Last 270 Years". United States Geological Survey (USGS). Retrieved May 1, 2007.
- 1 2 3 Pacyna E G, Pacyna J M, Steenhuisen F, Wilson S (2006). "Global anthropogenic mercury emission inventory for 2000". Atmos Environ 40 (22): 4048. doi:10.1016/j.atmosenv.2006.03.041.
- ↑ "What is EPA doing about mercury air emissions?". United States Environmental Protection Agency (EPA). Retrieved May 1, 2007.
- ↑ Solnit, R. (September–October 2006). "Winged Mercury and the Golden Calf". Orion Magazine. Retrieved December 3, 2007.
- ↑ Maprani, Antu C.; Al, Tom A.; MacQuarrie, Kerry T.; Dalziel, John A.; Shaw, Sean A.; Yeats, Phillip A. (2005). "Determination of Mercury Evasion in a Contaminated Headwater Stream". Environmental Science & Technology 39 (6): 1679. Bibcode:2005EnST...39.1679M. doi:10.1021/es048962j.
- ↑ "Indoor Air Mercury" (PDF). May 2003. Retrieved July 7, 2009.
- ↑ Fu, X., Feng, X. , Sommar, J., Wang, S., "A review of studies on atmospheric mercury in China", Science of the Total Environment, Volume 421-422, 1 April 2012, Pages 73-81, doi:10.1016/j.scitotenv.2011.09.089
- ↑ "Mercury-containing Products". United States Environmental Protection Agency (EPA). Retrieved May 1, 2007.
- 1 2 "Clean Air Mercury Rule". United States Environmental Protection Agency (EPA). Retrieved May 1, 2007.
- 1 2 "State of New Jersey et al., Petitioners vs. Environmental Protection Agency (Case No. 05-1097)]" (PDF). United States Court of Appeals for the District of Columbia Circuit. Argued December 6, 2007, Decided February 8, 2008. Retrieved May 30, 2008.
- ↑ "Introduction". Y-12 Mercury Task Force Files: A Guide to Record Series of the Department of Energy and its Contractors. United States Department of Energy. External link in
|work=(help) - ↑ "Minamata Disease The History and Measures". Ministry of the Environment, Government of Japan. Retrieved July 7, 2009.
- ↑ "Mercury: Spills, Disposal and Site Cleanup". Environmental Protection Agency. Retrieved August 11, 2007.
- ↑ "Safety data for mercuric sulphide". Oxford University. Retrieved July 7, 2009.
- ↑ Ngim, CH; Foo, SC; Boey, K.W.; Keyaratnam, J (1992). "Chronic neurobehavioral effects of elemental mercury in dentists". British Journal of Industrial Medicine 49 (11): 782–90. doi:10.1136/oem.49.11.782. PMC 1039326. PMID 1463679.
- ↑ Liang, YX; Sun, RK; Sun, Y; Chen, ZQ; Li, LH (1993). "Psychological effects of low exposure to mercury vapor: Application of computer-administered neurobehavioral evaluation system". Environmental Research 60 (2): 320–7. Bibcode:1993ER.....60..320L. doi:10.1006/enrs.1993.1040. PMID 8472661.
- ↑ McFarland, RB and Reigel, H (1978). "Chronic Mercury Poisoning from a Single Brief Exposure". J. Occup. Med. 20 (8): 532. doi:10.1097/00043764-197808000-00003.
- ↑ Mercury, Environmental Health Criteria monograph No. 001, Geneva: World Health Organization, 1976, ISBN 92-4-154061-3
- ↑ Inorganic mercury, Environmental Health Criteria monograph No. 118, Geneva: World Health Organization, 1991, ISBN 92-4-157118-7
- ↑ Bluhm, RE; et al. (1992). "Elemental Mercury Vapour Toxicity, Treatment, and Prognosis After Acute, Intensive Exposure in Chloralkali Plant Workers. Part I: History, Neuropsychological Findings and Chelator effects". Hum Exp Toxicol 11 (3): 201–10. doi:10.1177/096032719201100308. PMID 1352115.
- ↑ Bluhm, Re; Bobbitt, Rg; Welch, Lw; Wood, Aj; Bonfiglio, Jf; Sarzen, C; Heath, Aj; Branch, Ra (1992). "Elemental mercury vapour toxicity, treatment, and prognosis after acute, intensive exposure in chloralkali plant workers. Part I: History, neuropsychological findings and chelator effects.". Human & Experimental Toxicology 11 (3): 201–10. doi:10.1177/096032719201100308. PMID 1352115.
- ↑ Cocoros, G.; Cahn, P. H.; Siler, W. (1973). "Mercury concentrations in fish, plankton and water from three Western Atlantic estuaries" (PDF). Journal of Fish Biology 5 (6): 641–647. doi:10.1111/j.1095-8649.1973.tb04500.x.
- ↑ "Directive on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment". 2002/95/EC. Check date values in:
|date=(help) Article 4 Paragraph 1. e.g. "Member States shall ensure that, from July 1, 2006, new electrical and electronic equipment put on the market does not contain lead, mercury, cadmium, hexavalent chromium, polybrominated biphenyls (PBB) or polybrominated diphenyl ethers (PBDE)." - ↑ "Mercury compounds in European Union:". EIA Track. 2007. Retrieved May 30, 2008.
- ↑ Jones H. (July 10, 2007). "EU bans mercury in barometers, thermometers". Reuters. Retrieved May 30, 2008.
- ↑ "Norway to ban mercury". EU Business. December 21, 2007. Archived from the original on 2008-01-21. Retrieved May 30, 2008.
- ↑ Berg, T; Fjeld, E; Steinnes, E (2006). "Atmospheric mercury in Norway: contributions from different sources". The Science of the total environment 368 (1): 3–9. doi:10.1016/j.scitotenv.2005.09.059. PMID 16310836.
- ↑ "Mercury: Laws and regulations". United States Environmental Protection Agency. April 16, 2008. Retrieved May 30, 2008.
- ↑ "Reductions in Mercury Emissons". International Joint Commission on the Great Lakes.