Mefway (18F)
 | |
| Systematic (IUPAC) name | |
|---|---|
|
4-[(18F)fluoromethyl]-N-{2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl}-N-(pyridin-2-yl)cyclohexane-1-carboxamide | |
| Clinical data | |
| Pregnancy category |
|
| Legal status |
|
| Identifiers | |
| CAS Number | 943962-60-5 |
| ATC code | None |
| PubChem | CID 11963740 |
| ChemSpider | 10137857 |
| Chemical data | |
| Formula | C26H35FN4O2 |
| Molar mass | 453.583 g/mol |
| |
| |
Mefway is a serotonin 5-HT1A receptor antagonist used in medical research, usually in the form of mefway (18F) as a positron emission tomography (PET) radiotracer.[1]
Chemistry
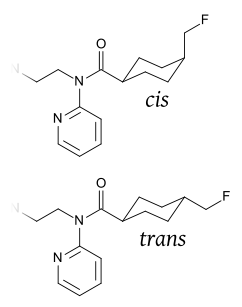
Mefway is closely related to the research compound WAY-100,635. The compound adds a fluoromethyl group to the cyclohexyl ring of WAY-100,635 and it is effectively prepared with automation module.[2] There are two isomers with regard to the cyclohexane ring, of which the trans conformation has the higher 5-HT1A specificity.[3]
Animal PET studies
In one study the uptake and retention of mefway (18F) was found to be similar to that found for 11C-WAY-100,635. Head-to-head comparison of mefway (18F) and 11C-WAY-100,635 have been evaluated. Since 11C-WAY-100,635 is the current ‘gold standard’ and difficult to synthesize, a suitable fluorine-18 replacement as in mefway is highly desired.[4] In addition, mefway (18F) showed comparable brain uptake and the target-to-reference ratios compared to fcway(18F)[5]
The ability to separately measure dissociation constant, KD and receptor density Bmax has been shown to be of potential value rather than simply comparing binding potential, BPND. Multiple injection mefway PET experiments can be used for the in-vivo measurement of 5-HT1A receptor density.[6]
Imaging studies of mefway on in vivo and ex vivo rat brains indicate that the substance binds to the known 5-HT1A receptor regions including the dorsal raphe. These findings support that the dorsal raphe is measurable in rat PET studies.[7] Mefway (18F) undergoes in vivo defluorination in rodent brain and this phenomena was effectively suppressed by cytochrome P450 inhibitor (i.e. fluconazole). [8] Animal models of Parkinson's disease and the acute physical stress model exhibited significant decrement of binding potential in the hippocampus [9][10]
Human PET studies
First-in-human studies have shown in vivo stability of mefway (18F) and its localization to 5-HT1A receptor-rich regions in the human brain, including the raphe nucleus.[11] Mefway (18F) is highly selective for the human serotonin 5-HT1A receptor and may therefore may be used to quantify serotonin 5-HT1A receptor distribution in brain regions for the study of various central nervous system disorders.[12]
References
- ↑ Saigal, N.; Pichika, R.; Easwaramoorthy, B.; Collins, D.; Christian, B. T.; Shi, B.; Narayanan, T. K.; Potkin, S. G.; Mukherjee, J. (2006). "Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, 18F-labeled mefway, in rodents and imaging by PET in a nonhuman primate". Journal of nuclear medicine 47 (10): 1697–1706. PMID 17015907.
- ↑ Choi, JY; Kim, CH; Ryu, YH; Seo, YB; Truong, P; Kim, EJ; Choi, TH; Kang, J; Lee, M; Kim, DG; Lee, JD; Jeon, TJ (2013). "Optimization of the radiosynthesis of [18F]MEFWAY for imaging brain serotonin 1A receptors by using the GE TracerLab FXFN-Pro module.". Journal of labelled compounds & radiopharmaceuticals 56 (12): 589–94. doi:10.1002/jlcr.3067. PMID 24285234.
- ↑ Wooten, D.; Hillmer, A.; Murali, D.; Barnhart, T.; Schneider, M. L.; Mukherjee, J.; Christian, B. T. (2011). "An in vivo comparison of cis- and trans-[18F]mefway in the nonhuman primate". Nuclear Medicine and Biology 38 (7): 925–32. doi:10.1016/j.nucmedbio.2011.04.001. PMID 21741252.
- ↑ Wooten, D. W.; Moraino, J. D.; Hillmer, A. T.; Engle, J. W.; Dejesus, O. J.; Murali, D.; Barnhart, T. E.; Nickles, R. J.; Davidson, R. J.; Schneider, M. L.; Mukherjee, J.; Christian, B. T. (2011). "In vivo kinetics of \F-18]MEFWAY: A comparison with \C-11]WAY100635 and \F-18]MPPF in the nonhuman primate". Synapse 65 (7): 592–600. doi:10.1002/syn.20878. PMC 3080024. PMID 21484878.
- ↑ Choi, JY; Kim, BS; Kim, CH; Kim, DG; Han, SJ; Lee, K; Kim, KM; An, G; Choi, TH; Yoo, SD; Ryu, YH (2014). "Translational possibility of [18 F]Mefway to image serotonin 1A receptors in humans: Comparison with [18 F]FCWAY in rodents". Synapse 68 (12): 595–603. doi:10.1002/syn.21771. PMID 25056144.
- ↑ Wooten, D. W.; Hillmer, A. T.; Moirano, J. M.; Ahlers, E. O.; Slesarev, M.; Barnhart, T. E.; Mukherjee, J.; Schneider, M. L.; Christian, B. T. (2012). "Measurement of 5-HT1A receptor density and in-vivo binding parameters of \18F]mefway in the nonhuman primate". Journal of Cerebral Blood Flow & Metabolism 32 (8): 1546–1558. doi:10.1038/jcbfm.2012.43. PMC 3421091. PMID 22472611.
- ↑ Saigal, N.; Bajwa, A. K.; Faheem, S. S.; Coleman, R. A.; Pandey, S. K.; Constantinescu, C. C.; Fong, V.; Mukherjee, J. (2013). "Evaluation of serotonin 5-HT1Areceptors in rodent models using \18F]mefway PET". Synapse 67 (9): 596–608. doi:10.1002/syn.21665. PMC 3744326. PMID 23504990.
- ↑ Choi, JY; Kim, CH; Jeon, TJ; Kim, BS; Yi, CH; Woo, KS; Seo, YB; Han, SJ; Kim, KM; Yi, DI; Lee, M; Kim, DG; Kim, JY; Lee, KC; Choi, TH; An, G; Ryu, YH (December 2012). "Effective microPET imaging of brain 5-HT(1A) receptors in rats with [(18) F]MeFWAY by suppression of radioligand defluorination". Synapse 66 (12): 1015–23. doi:10.1002/syn.21607. PMID 22927318.
- ↑ Lee, M; Ryu, Y. H.; Cho, W. G.; Jeon, T. J.; Lyoo, C. H.; Kang, Y. W.; Lee, S. J.; Kim, C. H.; Kim, D. G.; Kang, J. H.; Seo, Y. B.; Yi, C. H.; Lee, K; Choi, T. H.; Choi, J. Y. (2014). "Dopaminergic neuron destruction reduces hippocampal serotonin 1A receptor uptake of trans-(18)FMefway". Applied Radiation and Isotopes 94: 30–4. doi:10.1016/j.apradiso.2014.06.016. PMID 25064461.
- ↑ Choi, J. Y.; Shin, S; Lee, M; Jeon, T. J.; Seo, Y; Kim, C. H.; Kim, D. G.; Yi, C. H.; Lee, K; Choi, T. H.; Kang, J. H.; Ryu, Y. H. (2014). "Acute physical stress induces the alteration of the serotonin 1A receptor density in the hippocampus". Synapse 68 (8): 363–8. doi:10.1002/syn.21748. PMID 24771590.
- ↑ Hillmer, A. T.; Wooten, D. W.; Bajwa, A. K.; Higgins, A. T.; Lao, P. J.; Betthauser, T. J.; Barnhart, T. E.; Rowley, H. A.; Stone, C. K.; Johnson, S. C.; Mukherjee, J; Christian, B. T. (2014). "First-in-Human Evaluation of 18F-Mefway, a PET Radioligand Specific to Serotonin-1A Receptors". Journal of Nuclear Medicine 55 (12): 1973–9. doi:10.2967/jnumed.114.145151. PMID 25453045.
- ↑ Mukherjee, J; Bajwa, A. K.; Wooten, D. W.; Hillmer, A. T.; Pan, M. L.; Pandey, S. K.; Saigal, N; Christian, B. T. (2015). "Comparative assessment of 18 F-Mefway as a serotonin 5-HT1A receptor PET imaging agent across species- rodents, nonhuman primates, and humans". Journal of Comparative Neurology: n/a. doi:10.1002/cne.23919. PMID 26509362.
| ||||||||||||||||||||||||||||||||||||||