Mefenamic acid
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
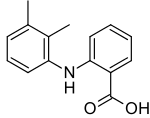
2-(2,3-dimethylphenyl)aminobenzoic acid | |
| Clinical data | |
| Trade names | Ponstel, Ponstan |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a681028 |
| Pregnancy category | |
| Legal status | |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Protein binding | 90% |
| Metabolism | Hepatic (CYP2C9) |
| Biological half-life | 2 hours |
| Excretion | Urine (66%), faeces (20-25%) |
| Identifiers | |
| CAS Number |
61-68-7 |
| ATC code | M01AG01 |
| PubChem | CID 4044 |
| IUPHAR/BPS | 2593 |
| DrugBank |
DB00784 |
| ChemSpider |
3904 |
| UNII |
367589PJ2C |
| KEGG |
D00151 |
| ChEBI |
CHEBI:6717 |
| ChEMBL |
CHEMBL686 |
| Chemical data | |
| Formula | C15H15NO2 |
| Molar mass | 241.285 g/mol |
| |
| |
| | |
Mefenamic acid is a member of the anthranilic acid derivatives (or fenamate) class of NSAID drugs, and is used to treat mild to moderate pain, including menstrual pain, and is sometimes used to prevent migraines associated with menstruation.[1][2] It is not widely used due to its side effects.[3][4]:334
It was discovered and brought to market by Parke-Davis in the 1960s under brandnames Ponstan, Ponalar, Ponstyl, and Ponstel. It became generic in the 1980s is available worldwide under many brand names.[5]
Medical use
Mefenamic acid is used to treat moderate pain and menstrual pain.[1] It is not widely used.[3][4]:334
There is evidence that supports the use of mefenamic acid for perimenstrual migraine headache prophylaxis, with treatment starting 2 days prior to the onset of flow or 1 day prior to the expected onset of the headache and continuing for the duration of menstruation.[2] A study also showed mefenamic acid to be a well tolerated analgesic for post-surgical pain in dental operations.[6]
Side effects
Mefenamic acid is recommended to be taken with food.[7]
Known mild side effects of mefenamic acid include headaches, nervousness and vomiting. Serious side effects may include diarrhea, hematemesis (vomiting blood), haematuria (blood in urine), blurred vision, skin rash, itching and swelling, sore throat and fever.[4]:334 It has been associated with acute liver damage.[3]
In 2008 the US label was updated with a warning concerning a risk of premature closure of the ductus arteriosus in pregnancy.[8]
Mechanism of action
Like other members of the anthranilic acid derivatives (or fenamate) class of NSAID drugs, it inhibits both isoforms of COX and prevents formation of prostaglandins.[3][9]
Synthesis
Analogous to fenamic acid, this compound may be synthesized from 2-chlorobenzoic acid and 2,3-dimethylaniline.[10]
History
Scientists led by Claude Winder from Parke-Davis invented mefenamic acid in 1961, along with fellow members of the class of anthranilic acid derivatives, flufenamic acid in 1963 and meclofenamate sodium in 1964.[11]:718 U.S. Patent 3,138,636 on the drug was issued in 1964.[12][13]:918–919
It was approved in the UK in 1963 as "Ponstan", in West Germany in 1964 as "Ponalar", and in France as "Ponstyl" and the US in 1967 as "Ponstel".[3][13]:918–919
Society and culture
Mefenamic acid is generic and is available worldwide under many brand names.[5]
In the USA, wholesale price of a week's supply of generic mefenamic acid has been quoted as $426.90 in 2014. Brand-name Ponstel is $571.70.[14] In contrast, in the UK, a weeks supply is £1.66, or £8.17 for branded Ponstan.[15] In the Philippines, 10 tablets of 500 mg generic mefenamic acid cost PHP39.00 (or the equivalent of $0.88USD)as of October 25, 2014.
See also
References
- 1 2 FDA Ponstel Label Updated February 19, 2008
- 1 2 Pringsheim, T.; Davenport, W. J.; Dodick, D. (2008). "Acute Treatment and Prevention of Menstrually Related Migraine Headache: Evidence-Based Review". Neurology 70 (17): 1555–1563. doi:10.1212/01.wnl.0000310638.54698.36. PMID 18427072.
- 1 2 3 4 5 NIH LiverTox Database Mefenamic Acid Last updated June 23, 2015. Page accessed July 3, 2015
- 1 2 3 Jeffrey K. Aronson. Meyler's Side Effects of Analgesics and Anti-inflammatory Drugs. Elsevier, 2009 ISBN 9780080932941
- 1 2 Drugs.com drugs.com international listings for mefenamic acid Page accessed July 3, 2015
- ↑ Rowe, N. H.; Cudmore, C. L.; Turner, J. L. (1981-06-01). "Control of pain by mefenamic acid following removal of impacted molar. A double-blind, placebo-controlled study". Oral Surgery, Oral Medicine, and Oral Pathology 51 (6): 575–580. ISSN 0030-4220. PMID 7019803.
- ↑ "Side effects for Mefenamic Acid". Medline Plus. National Institutes of Health.
- ↑ FDA March 2008 FDA advisory
- ↑ Prusakiewicz JJ et al. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry. 2009 Aug 11;48(31):7353-5 PMID 19603831 PMC 2720641
- ↑ Trinus, F. P.; Mokhort, N. A.; Yagupol'skii, L. M.; Fadeicheva, A. G.; Danilenko, V. S.; Ryabukha, T. K.; Fialkov, Yu. A.; Kirichek, L. M.; Endel'man, É. S.; Get'man, G. A. (1977). "Mefenamic acid — A Nonsteroid Antiinflammatory Agent". Pharmaceutical Chemistry Journal 11 (12): 1706–1711. doi:10.1007/BF00778304.
- ↑ Whitehouse M. Drugs to Treat Inflammation: A Historical Overview. pp 707-729 in Frontiers in Medicinal Chemistry , Volume 4. Eds Rahman A, et al. Bentham Science Publishers, 2009 ISBN 9781608052073
- ↑ US Patent 3,138,636
- 1 2 Marshall Sittig Pharmaceutical Manufacturing Encyclopedia Volume 1 A-K Second Edition, Reprint Edition. Noyes Publications, 1988
- ↑ Drugs for Osteoarthritis. The Medical Letter, 56(1450):80-84, September 2014
- ↑ https://www.medicinescomplete.com/mc/bnf/current/PHP6487-mefenamic-acid-non-proprietary.htm accessed 19th sept 2014
Sources
- MedlinePlus Drug Information: Mefenamic Acid. Last accessed September 28, 2005.
- Ponstel Pharmacology, Pharmacokinetics, Studies, Metabolism - Mefenamic Acid - RxList Monographs. Last accessed September 28, 2005.
- Consumption of NSAIDs and the Development of Congestive Heart Failure in Elderly Patients
- NSAIDs May Increase Risk for Worsening Heart Failure
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||