Chloramphenicol
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
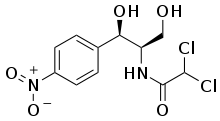
2,2-dichloro-N-[1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide | |
| Clinical data | |
| Trade names | Pentamycetin, Chloromycetin[1] |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a608008 |
| Licence data | US FDA:link |
| Pregnancy category | |
| Legal status | |
| Routes of administration | Topical (eye drops), by, IV, IM |
| Pharmacokinetic data | |
| Bioavailability | 75–90% |
| Protein binding | 60% |
| Metabolism | Liver |
| Biological half-life | 1.6-3.3 hours |
| Excretion | Kidney (5-15%), faeces (4%) |
| Identifiers | |
| CAS Number |
56-75-7 |
| ATC code | D06AX02 D10AF03 G01AA05 J01BA01 S01AA01 S02AA01 S03AA08 QJ51BA01 |
| PubChem | CID 298 |
| DrugBank |
DB00446 |
| ChemSpider |
5744 |
| UNII |
66974FR9Q1 |
| KEGG |
D00104 |
| ChEBI |
CHEBI:17698 |
| ChEMBL |
CHEMBL130 |
| Chemical data | |
| Formula | C11H12Cl2N2O5 |
| Molar mass | 323.1320 g/mol |
| |
| |
| (verify) | |
Chloramphenicol is an antibiotic useful for the treatment of a number of bacterial infections.[2] This includes meningitis, plague, cholera, and typhoid fever. Its use is only recommended when safer antibiotics cannot be used. Monitoring both blood levels of the medication and blood cell levels every two days is recommended during treatment.[2] It is available intravenously, by mouth, and as an eye ointment.[1][3]
Common side effects include bone marrow suppression, nausea, and diarrhea. The bone marrow suppression may result in death. To reduce the risk of side effects treatment duration should be as short as possible. In those with liver or kidney problems dosing may require decreasing. In young children a condition known as gray baby syndrome may occur which results in a swollen stomach and low blood pressure.[2] Its use near the end of pregnancy and during breastfeeding is typically not recommended.[4] Chloramphenicol is a broad-spectrum antibiotic that typically stops bacterial growth by stopping the making of protein.[2]
Chloramphenical was discovered in 1947.[5] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[6] It is available as a generic medication.[2] The wholesale cost of an intravenous dose is about 0.40 to 1.90 USD.[7] In the United States it is very expensive.[8] Global issues relating to bacterial resistance have revived interest in its use.[9]
Medical uses
The original indication of chloramphenicol was in the treatment of typhoid, but the now almost universal presence of multiple drug-resistant Salmonella typhi has meant it is seldom used for this indication except when the organism is known to be sensitive. Chloramphenicol may be used as a second-line agent in the treatment of tetracycline-resistant cholera.
Because of its excellent blood-brain barrier penetration (far superior to any of the cephalosporins), chloramphenicol remains the first-choice treatment for staphylococcal brain abscesses. It is also useful in the treatment of brain abscesses due to mixed organisms or when the causative organism is not known.
Chloramphenicol is active against the three main bacterial causes of meningitis: Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. In the West, chloramphenicol remains the drug of choice in the treatment of meningitis in patients with severe penicillin or cephalosporin allergy and general practitioners are recommended to carry intravenous chloramphenicol in their bag. In low-income countries, the WHO recommend oily chloramphenicol as first-line to treat meningitis.
Chloramphenicol has been used in the U.S. in the initial empirical treatment of children with fever and a petechial rash, when the differential diagnosis includes both Neisseria meningitidis septicaemia and Rocky Mountain spotted fever, pending the results of diagnostic investigations.
Chloramphenicol is also effective against Enterococcus faecium, which has led to its being considered for treatment of vancomycin-resistant enterococcus.
The drug should be discontinued upon appearance of reticulocytopenia, leukopenia, thrombocytopenia, anemia, or any other abnormal blood study findings attributable to chloramphenicol.[10]
Spectrum of activity
Chloramphenicol has a broad spectrum of activity and has been effective in treating ocular infections caused by a number of bacteria including Staphylococcus aureus, Streptococcus pneumoniae, and Escherichia coli. It is not effective against Pseudomonas aeruginosa. The following susceptibility data represent the minimum inhibitory concentration for a few medically significant organisms.[11]
- Escherichia coli: 0.015-10,000 μg/ml
- Staphylococcus aureus: 0.06 μg/ml - > 128 μg/ml
- Streptococcus pneumoniae: 2-16 μg/ml
Each of these concentrations is dependent upon the bacterial strain being targeted. Some strains of E. coli, for example, show spontaneous emergence of chloramphenicol resistance.[12][13]
Resistance
Three mechanisms of resistance to chloramphenicol are known: reduced membrane permeability, mutation of the 50S ribosomal subunit, and elaboration of chloramphenicol acetyltransferase. It is easy to select for reduced membrane permeability to chloramphenicol in vitro by serial passage of bacteria, and this is the most common mechanism of low-level chloramphenicol resistance. High-level resistance is conferred by the cat-gene; this gene codes for an enzyme called chloramphenicol acetyltransferase, which inactivates chloramphenicol by covalently linking one or two acetyl groups, derived from acetyl-S-coenzyme A, to the hydroxyl groups on the chloramphenicol molecule. The acetylation prevents chloramphenicol from binding to the ribosome. Resistance-conferring mutations of the 50S ribosomal subunit are rare.
Chloramphenicol resistance may be carried on a plasmid that also codes for resistance to other drugs. One example is the ACCoT plasmid (A=ampicillin, C=chloramphenicol, Co=co-trimoxazole, T=tetracycline), which mediates multiple-drug resistance in typhoid (also called R factors).
Currently, some Enterococcus faecium and Pseudomonas aeruginosa strains are resistant to chloramphenicol. Some Veillonella spp. and Staphylococcus capitis strains have also developed resistance to chloramphenicol to varying degrees.[14]
Adverse effects
Aplastic anemia
The most serious side effect of chloramphenicol treatment is aplastic anaemia.[15] This effect is rare and is generally fatal. No treatment is available and no way exists to predict who may or may not get this side effect. The effect usually occurs weeks or months after treatment has been stopped, and a genetic predisposition may be involved.[16] It is not known whether monitoring the blood counts of patients can prevent the development of aplastic anaemia, but patients are recommended to have a baseline blood count with a repeat blood count every few days while on treatment.[17] Chloramphenicol should be discontinued if the complete blood count drops below 2.5 x 10 cells/l. The highest risk is with oral chloramphenicol[18] (affecting 1 in 24,000–40,000)[19] and the lowest risk occurs with eye drops (affecting less than one in 224,716 prescriptions).[20]
Thiamphenicol, a related compound with a similar spectrum of activity, is available in Italy and China for human use, and has never been associated with aplastic anaemia . Thiamphenicol is available in the U.S. and Europe as a veterinary antibiotic, but is not approved for use in humans.
Bone marrow suppression
Chloramphenicol may cause bone marrow suppression during treatment; this is a direct toxic effect of the drug on human mitochondria.[21] This effect manifests first as a fall in hemoglobin levels, which occurs quite predictably once a cumulative dose of 20 g has been given. The anaemia is fully reversible once the drug is stopped and does not predict future development of aplastic anaemia. Studies in mice have suggested existing marrow damage may compound any marrow damage resulting from the toxic effects of chloramphenicol.[22]
Leukemia
Leukemia, a cancer of the blood or bone marrow, is characterized by an abnormal increase of immature white blood cells. The risk of childhood leukemia is increased, as demonstrated in a Chinese case-controlled study,[23] and the risk increases with length of treatment.
Gray baby syndrome
Intravenous chloramphenicol use has been associated with the so-called gray baby syndrome.[24] This phenomenon occurs in newborn infants because they do not yet have fully functional liver enzymes (i.e. UDP-glucuronyl transferase), so chloramphenicol remains unmetabolized in the body.[25] This causes several adverse effects, including hypotension and cyanosis. The condition can be prevented by using the drug at the recommended doses, and monitoring blood levels.[26][27][28]
Hypersensitivity reactions
Fever, macular and vesicular rashes, angioedema, urticaria, and anaphylaxis may occur. Herxheimer’s reactions have occurred during therapy for typhoid fever.[10]
Neurotoxic reactions
Headache, mild depression, mental confusion, and delirium have been described in patients receiving chloramphenicol. Optic and peripheral neuritis have been reported, usually following long-term therapy. If this occurs, the drug should be promptly withdrawn.[10]
Pharmacokinetics
Chloramphenicol is extremely lipid-soluble; it remains relatively unbound to protein and is a small molecule. It has a large apparent volume of distribution and penetrates effectively into all tissues of the body, including the brain. Distribution is not uniform, with highest concentrations found in the liver and kidney, with lowest in the brain and cerebrospinal fluid.[10] The concentration achieved in brain and cerebrospinal fluid is around 30 to 50% of the overall average body concentration, even when the meninges are not inflamed; this increases to as high as 89% when the meninges are inflamed.
Chloramphenicol increases the absorption of iron.[29]
Use in special populations
Chloramphenicol is metabolized by the liver to chloramphenicol glucuronate (which is inactive). In liver impairment, the dose of chloramphenicol must therefore be reduced. No standard dose reduction exists for chloramphenicol in liver impairment, and the dose should be adjusted according to measured plasma concentrations.
The majority of the chloramphenicol dose is excreted by the kidneys as the inactive metabolite, chloramphenicol glucuronate. Only a tiny fraction of the chloramphenicol is excreted by the kidneys unchanged. Plasma levels should be monitored in patients with renal impairment, but this is not mandatory. Chloramphenicol succinate ester (an intravenous prodrug form) is readily excreted unchanged by the kidneys, more so than chloramphenicol base, and this is the major reason why levels of chloramphenicol in the blood are much lower when given intravenously than orally.
Chloramphenicol passes into breast milk, so should therefore be avoided during breast feeding, if possible.[30]
Dose monitoring
Plasma levels of chloramphenicol must be monitored in neonates and patients with abnormal liver function. Plasma levels should be monitored in all children under the age of four, the elderly, and patients with renal failure. Because efficacy and toxicity of chloramphenicol are associated with a maximum serum concentration, peak levels (one hour after the intravenous dose is given) should be 10-20 µg/ml with toxicity > 40 µg/ml; trough levels (taken immediately before a dose) should be 5-10 µg/ml.[31][32]
Drug interactions
Administration of chloramphenicol concomitantly with bone marrow depressant drugs is contraindicated, although concerns over aplastic anaemia associated with ocular chloramphenicol have largely been discounted.[33]
Chloramphenicol is a potent inhibitor of the cytochrome P450 isoforms CYP2C19 and CYP3A4 in the liver.[34] Inhibition of CYP2C19 causes decreased metabolism and therefore increased levels of, for example, antidepressants, antiepileptics, proton pump inhibitors, and anticoagulants if they are given concomitantly. Inhibition of CYP3A4 causes increased levels of, for example, calcium channel blockers, immunosuppressants, chemotherapeutic drugs, benzodiazepines, azole antifungals, tricyclic antidepressants, macrolide antibiotics, SSRIs, statins, cardiac antiarrhythmics, antivirals, anticoagulants, and PDE5 inhibitors.[10][35]
Drug antagonistic
Chloramphenicol is antagonistic with most Cephalosporins and using both together should be avoided in the treatment of infections.[36]
Mechanism of action
Chloramphenicol is a bacteriostatic by inhibiting protein synthesis. It prevents protein chain elongation by inhibiting the peptidyl transferase activity of the bacterial ribosome. It specifically binds to A2451 and A2452 residues[37] in the 23S rRNA of the 50S ribosomal subunit, preventing peptide bond formation.[38] While chloramphenicol and the macrolide class of antibiotics both interact with ribosomes, chloramphenicol is not a macrolide. It directly interferes with substrate binding, whereas macrolides sterically block the progression of the growing peptide.[39][40][41]
History
Chloramphenicol was originally derived from the bacterium Streptomyces venezuelae, isolated by David Gottlieb, and introduced into clinical practice in 1949, under the trade name Chloromycetin. It was the first antibiotic to be manufactured synthetically on a large scale.
The topical formulation of chloramphenicol was commonly used as eye drops as first-line treatment of conjunctivitis. The first fatality from eye drops was reported in 1955.[42]
In 2007, the accumulation of reports associating aplastic anemia and blood dyscrasia with chloramphenicol eye drops lead to the classification of “probable” according to World Health Organization criteria, based on the known published case reports and the spontaneous reports submitted to the National Registry of Drug-Induced Ocular Side Effects.[43]
Society and culture
Cost
In many areas of the world an intravenous dose is about 0.40 to 1.90 USD.[7] In the United States it is very expensive.[8]
Names
Chloramphenicol is available as a generic worldwide under many brandnames[44] and also under various generic names in eastern Europe and Russia, including chlornitromycin, levomycetin, and chloromycetin; the racemate is known as synthomycetin.[45]
Formulations

Chloramphenicol is available as a capsules or as a liquid . In some countries, it is sold as chloramphenicol palmitate ester (CPE). CPE is inactive, and is hydrolysed to active chloramphenicol in the small intestine. No difference in bioavailability is noted between chloramphenicol and CPE.
Manufacture of oral chloramphenicol in the U.S. stopped in 1991, because the vast majority of chloramphenicol-associated cases of aplastic anaemia are associated with the oral preparation. No oral formulation of chloramphenicol is now available in the U.S.
In molecular biology, chloramphenicol is prepared in ethanol.
Intravenous
The intravenous (IV) preparation of chloramphenicol is the succinate ester, because pure chloramphenicol does not dissolve in water. This creates a problem: Chloramphenicol succinate ester is an inactive prodrug and must first be hydrolysed to chloramphenicol; however, the hydrolysis process is often incomplete, and 30% of the dose is lost and removed in the urine. Serum concentrations of IV chloramphenicol are only 70% of those achieved when chloramphenicol is given orally.[46] For this reason, the dose needs to be increased to 75 mg/kg/day when administered IV to achieve levels equivalent to the oral dose.[47]
Oily
Oily chloramphenicol (or chloramphenicol oil suspension) is a long-acting preparation of chloramphenicol first introduced by Roussel in 1954; marketed as Tifomycine, it was originally used as a treatment for typhoid. Roussel stopped production of oily chloramphenicol in 1995; the International Dispensary Association has manufactured it since 1998, first in Malta and then in India from December 2004.[48]
Oily chloramphenicol is recommended by the World Health Organization as the first-line treatment of meningitis in low-income countries, and appears on the WHO essential drugs list. It was first used to treat meningitis in 1975[49] and numerous studies since have demonstrated its efficacy.[50][51][52] It is the cheapest treatment available for meningitis (US$5 per treatment course, compared to US$30 for ampicillin and US$15 for five days of ceftriaxone). It has the great advantage of requiring only a single injection, whereas ceftriaxone is traditionally given daily for five days. This recommendation may yet change, now that a single dose of ceftriaxone (cost US$3) has been shown to be equivalent to one dose of oily chloramphenicol.[53]
Eye drops
Chloramphenicol is still widely used in topical preparations (ointments and eye drops) for the treatment of bacterial conjunctivitis. Isolated case reports of aplastic anaemia following use of chloramphenicol eyedrops exist, but the risk is estimated to be less than one in 224,716 prescriptions.[20] In Mexico, this is the treatment used prophylactically in newborns.
Veterinary uses
Although its use in veterinary medicine is highly restricted, chloramphenicol still has some important veterinary uses.[54] It is currently considered the most useful treatment of chlamydial disease in koalas.[55][56] The pharmacokinetics of chloramphenicol have been investigated in koalas.[57]
Although unpublished, recent research suggests chloramphenicol could also be applied to frogs to prevent their widespread destruction from fungal infections.[58] It has recently been discovered to be a life-saving cure for chytridiomycosis in amphibians.[59] Chytridiomycosis is a fungal disease, blamed for the extinction of one-third of the 120 frog species lost since 1980.
References
- 1 2 Woods, Adrienne L. (2008). Delmar nurse's drug handbook. (2009 ed.). Clifton Park, N.Y.: Delmar. p. 296. ISBN 9781428361065.
- 1 2 3 4 5 "Chloramphenicol". The American Society of Health-System Pharmacists. Retrieved Aug 1, 2015.
- ↑ Truven Health Micromedex. "chloramphenicol (Oral route, Intravenous route, Injection route)". Retrieved 26 August 2015.
- ↑ "Chloramphenicol Pregnancy and Breastfeeding Warnings". Multum Information Services. Retrieved 26 August 2015.
- ↑ Oxford Handbook of Infectious Diseases and Microbiology. OUP Oxford. 2009. p. 56. ISBN 9780191039621.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- 1 2 "Chloramphenicol". International Drug Price Indicator Guide. Retrieved 26 August 2015.
- 1 2 Hamilton, Richard J. (2013). Tarascon pocket pharmacopoeia. (14 ed.). Burlington, MA.: Jones & Bartlett Learning. p. 89. ISBN 9781449673635.
- ↑ Falagas, M. E.; Grammatikos, A. P.; Michalopoulos, A. (October 2008). "Potential of old-generation antibiotics to address current need for new antibiotics". Expert Review of Anti Infective Therapy 6 (5): 593–600. doi:10.1586/14787210.6.5.593. PMID 18847400.
- 1 2 3 4 5 "Drug Insert from DailyMed". Retrieved 18 April 2014.
- ↑ http://antibiotics.toku-e.com/antimicrobial_507.html
- ↑ Carone, BR; Xu, T; Murphy, KC; Marinus, MG (Jan 2014). "High incidence of multiple antibiotic resistant cells in cultures of in enterohemorrhagic Escherichia coli O157:H7.". Mutation research 759: 1–8. doi:10.1016/j.mrfmmm.2013.11.008. PMID 24361397.
- ↑ Moore, AM; Patel, S; Forsberg, KJ; Wang, B; Bentley, G; Razia, Y; Qin, X; Tarr, PI; Dantas, G (2013). "Pediatric fecal microbiota harbor diverse and novel antibiotic resistance genes.". PLoS ONE 8 (11): e78822. doi:10.1371/journal.pone.0078822. PMC 3827270. PMID 24236055.
- ↑ "Chloramphenicol spectrum of bacterial susceptibility and Resistance" (PDF). Retrieved 15 May 2012.
- ↑ Rich, M.; Ritterhoff, R.; Hoffmann, R. (December 1950). "A fatal case of aplastic anemia following chloramphenicol (chloromycetin) therapy". Annals of Internal Medicine 33 (6): 1459–1467. doi:10.7326/0003-4819-33-6-1459. PMID 14790529.
- ↑ Nagao, T.; Mauer, A. (July 1969). "Concordance for drug-induced aplastic anemia in identical twins". New England Journal of Medicine 281 (1): 7–11. doi:10.1056/NEJM196907032810102. PMID 5785754.
- ↑ Hammett-Stabler, C.; Johns, T. (May 1988). "Laboratory guidelines for monitoring of antimicrobial drugs". Clinical Chemistry 44 (5): 1129–1140. PMID 9590397.
- ↑ Holt, R. (1967). "The bacterial degradation of chloramphenicol". Lancet 289 (7502): 1259–1260. doi:10.1016/S0140-6736(67)92720-1. PMID 4165044.
- ↑ Wallerstein, R.; Condit, P.; Kasper, C.; Brown, J.; Morrison, F. (June 1969). "Statewide study of chloramphenicol therapy and fatal aplastic anemia". JAMA 208 (11): 2045–2050. doi:10.1001/jama.208.11.2045. PMID 5818983.
- 1 2 Lancaster, T.; Stewart, A. M.; Jick, H. (1998). "Risk of serious haematological toxicity with use of chloramphenicol eye drops in a British general practice database". British Medical Journal 316 (7132): 667. doi:10.1136/bmj.316.7132.667. PMC 28473. PMID 9522792.
- ↑ Yunis AA (September 1989). "Chloramphenicol toxicity: 25 years of research". Am. J. Med. 87 (3N): 44N–48N. PMID 2486534.
- ↑ Morley, Alec; Trainor, Kevin; Remes, Judith (1 April 1976). "Residual Marrow Damage: Possible Explanation for Idiosyncrasy to Chloramphenicol". British Journal of Haematology 32 (4): 525–532. doi:10.1111/j.1365-2141.1976.tb00955.x.
- ↑ Shu, X.; Gao, Y.; Linet, M.; Brinton, L.; Gao, R.; Jin, F.; Fraumeni, J. (October 1987). "Chloramphenicol use and childhood leukaemia in Shanghai". Lancet 2 (8565): 934–937. doi:10.1016/S0140-6736(87)91420-6. PMID 2889862.
- ↑ McIntyre, J.; Choonara, I. (2004). "Drug toxicity in the neonate". Biology of the Neonate 86 (4): 218–221. doi:10.1159/000079656. PMID 15249753.
- ↑ Piñeiro-Carrero, V.; Piñeiro, E. (2004). "Liver" (pdf). Pediatrics 113 (4 Supplement): 1097–1106. PMID 15060205.
- ↑ Feder, H. (1986). "Chloramphenicol: what we have learned in the last decade". Southern Medical Journal 79 (9): 1129–1134. doi:10.1097/00007611-198609000-00022. PMID 3529436.
- ↑ Mulhall, A.; de Louvois J.; Hurley, R. (1983). "Chloramphenicol toxicity in neonates: its incidence and prevention". British Medical Journal (Clinical Research Edition) 287 (6403): 1424–1427. doi:10.1136/bmj.287.6403.1424. PMC 1549666. PMID 6416440.
- ↑ Forster, J.; Hufschmidt, C.; Niederhoff, H.; Künzer, W. (1985). "[Need for the determination of chloramphenicol levels in the treatment of bacterial-purulent meningitis with chloramphenicol succinate in infants and small children]". Monatsschrift Kinderheilkunde (in German) 133 (4): 209–213. PMID 4000136.
- ↑ Harold M. Silverman, Pharm.D. (editor-in-chief), ed. (2006). "Iron Supplements". Pill Book, The (12th revised ed.). New York: Bantam Dell. pp. 593–596. ISBN 978-0-553-58892-7.
- ↑ kidsgrowth.org --> Drugs and Other Substances in Breast Milk Retrieved on June 19, 2009
- ↑ Hammett-Stabler, C; Johns, T. (May 1988). "Laboratory guidelines for monitoring of antimicrobial drugs". Clinical Chemistry 44 (5): 1129–1140. PMID 9590397.
- ↑ "Chloramphenicol (Lexi-Drugs)". Lexi-Comp Online. Retrieved 18 April 2014.
- ↑ June 2005. Royal Pharmaceutical Society of Great Britain (RPSGB)
- ↑ Park, J. Y.; Kim, K. A.; Kim, S. L. (November 2003). "Chloramphenicol Is a Potent Inhibitor of Cytochrome P450 Isoforms CYP2C19 and CYP3A4 in Human Liver Microsomes". Antimicrobial Agents and Chemotherapy 47 (11): 3464–3469. doi:10.1128/AAC.47.11.3464-3469.2003. PMC 253795. PMID 14576103.
- ↑ "Facts for prescribers (Fakta för förskrivare)" (in Swedish). FASS - Swedish National Drug Formulary.
- ↑ "Antagonistic effect of chloramphenicol in combination with cefotaxime or ceftriaxone". Retrieved August 25, 2014.
- ↑ Schifano JM, Edifor R, Sharp JD; et al. (May 2013). "Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23S rRNA at the ribosomal A site". Proceedings of the National Academy of Sciences of the United States of America 110: 8501–6. doi:10.1073/pnas.1222031110. PMC 3666664. PMID 23650345.
- ↑ "Chloramphenicol". The Merck Manual. Merck.
- ↑ Jardetzky, O. (July 1963). "Studies on the Mechanism of Action of Chloramphenicol - The Conformation of Chloramphenicol in Solution" (pdf). The Journal of Biological Chemistry 238 (7): 2498–2508. PMID 13957484.
- ↑ Wolfe, A. D.; Hahn, F. E. (1965). "Mode of Action of Chloramphenicol. IX. Effects of Chloramphenicol Upon a Ribosomal Amino Acid Polymerization System and Its Binding to Bacterial Ribosome". Biochimica et Biophysica Acta 95: 146–155. doi:10.1016/0005-2787(65)90219-4. PMID 14289020.
- ↑ Hahn, F. E.; Wisseman, C. L. Jr.; Hopps, H. E. (1955). "Mode of Action of Chloramphenicol III. : Action of Chloramphenicol on Bacterial Energy Metabolism". Journal of Bacteriology 69 (2): 215–223. PMC 357505. PMID 14353832.
- ↑ R.L. Rosenthal, A. Blackman (1965). "Bone-marrow hypoplasia following use of chloramphenicol eye drops". JAMA 191 (3): 136–137. doi:10.1001/jama.1965.03080020064025.
- ↑ Fraunfelder, F.; Fraunfelder, F (September 2013). "Restricting Topical Ocular Chloramphenicol Eye Drop Use in the United States. Did We Overreact?". American Journal of Ophtalmology 156 (3): 420–422. doi:10.1016/j.ajo.2013.05.004. PMID 23953152.
- ↑ Drugs.com drugs.com international listings for chloramphenicol Page accessed July 9, 2015
- ↑ The Great Soviet Encyclopedia, 3rd Edition, 1970-1979 (3 ed.). The Gale Group, Inc. Retrieved 10 July 2015.
- ↑ Glazko, A. J.; Dill, W. A.; Kinkel, A. W. (1977). "Absorption and excretion of parenteral doses of chloramphenicol sodium succinate in comparison with per oral doses of chloramphenicol (abstract)". Clinical Pharmacological Therapy 21: 104.
- ↑ Bhutta, Z.; Niazi, S.; Suria, A. (March–April 1992). "Chloramphenicol Clearance in Typhoid Fever: Implications for Therapy". Indian Journal of Pediatry 59 (2): 213–219. doi:10.1007/BF02759987. PMID 1398851.
- ↑ Lewis, R. F.; Dorlencourt, F.; Pinel, J. (1998). "Long-acting oily Chloramphenicol for Meningococcal Meningitis". Lancet 352 (9130): 823. doi:10.1016/S0140-6736(05)60723-4. PMID 9737323.
- ↑ Rey, M.; Ouedraogo, L.; Saliou, P.; Perino, L. (1976). "Traitement minute de la méningite cérébrospinale épidémique par injection intramusculaire unique de chloramphénicol (suspension huileuse)". Médecine et Maladies Infectieuses (in French) 6 (4): 120–124. doi:10.1016/S0399-077X(76)80134-5.
- ↑ Wali, S.; Macfarlane, J.; Weir, W.; Cleland, P.; Ball, P.; Hassan-King, M.; Whittle, H.; Greenwood, B. (1979). "Single injection treatment of meningococcal meningitis. 2. Long-acting chloramphenicol". Transactions of the Royal Society for Tropical Medicine and Hygiene 73 (6): 698–702. doi:10.1016/0035-9203(79)90024-5. PMID 538813.
- ↑ Puddicombe, J.; Wali, S.; Greenwood, B. (1984). "A field trial of a single intramuscular injection of long-acting chloramphenicol in the treatment of meningococcal meningitis". Transactions of the Royal Society for Tropical Medicine and Hygiene 78 (3): 399–403. doi:10.1016/0035-9203(84)90132-9. PMID 6464136.
- ↑ Pécoulm B.; Varaine, F.; Keita, M.; Soga, G.; Djibo, A.; Soula, G.; Abdou, A.; Etienne, J.; Rey, M. (October 1991). "Long-acting chloramphenicol versus intravenous ampicillin for treatment of bacterial meningitis". Lancet 338 (8771): 862–866. doi:10.1016/0140-6736(91)91511-R. PMID 1681224.
- ↑ Nathan, N.; Borel, T.; Djibo, A. (2005). "Ceftriaxone as effective as long-acting chloramphenicol in short-course treatment of meningococcal meningitis during epidemics: a randomised non-inferiority study". Lancet 366 (9482): 308–313. doi:10.1016/S0140-6736(05)66792-X. PMID 16039333.
- ↑ "Chloramphenicol and Congeners". Merck Manuals. Retrieved 31 October 2014.
- ↑ Govendir, M (16 May 2011). "Plasma concentrations of chloramphenicol after subcutaneous administration to koalas (Phascolarctos cinereus) with chlamydiosis". Journal of Veterinary Pharmacology and Therapeutics 35: 147–154. doi:10.1111/j.1365-2885.2011.01307.x.
- ↑ Griffith, J. (2012). "Diagnosis, treatment and outcomes for koala chlamydiosis at a rehabilitation facility (1995–2005)". Australian Veterinary Journal 90 (90): 457–463. doi:10.1111/j.1751-0813.2012.00963.x. PMID 23106328.
- ↑ Black, Lisa. "Pharmacokinetics of chloramphenicol following administration of intravenous and subcutaneous chloramphenicol sodium succinate, and subcutaneous chloramphenicol, to koalas ( Phascolarctos cinereus )". Journal of Veterinary Pharmacology and Therapeutics 36: 478–485. doi:10.1111/jvp.12024.
- ↑ Griggs, K. (2007-10-30). "Frog killer fungus 'breakthrough'". BBC News.
- ↑ Poulter, R. T. M.; Busby, J. N.; Bishop, P. J.; Butler, M. I.; Speare, R. "Chloramphenicol cures chytridiomycosis" (pdf). NZ Frogs.
Further reading
- Jardetzky, O. (1963). "Studies on the Mechanism of Action of Chloramphenicol". Journal of Biological Chemistry 238 (7): 2498–2508.
External links
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||
|