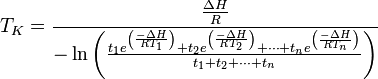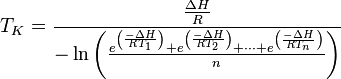Mean kinetic temperature
Mean kinetic temperature (MKT) is a simplified way of expressing the overall effect of temperature fluctuations during storage or transit of perishable goods. The MKT is widely used in the pharmaceutical industry.
The mean kinetic temperature can be expressed as:
Where:
-
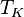 is the mean kinetic temperature in kelvins
is the mean kinetic temperature in kelvins -
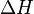 is the activation energy
is the activation energy -
 is the gas constant
is the gas constant -
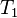 to
to 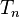 are the temperatures at each of the sample points in kelvins
are the temperatures at each of the sample points in kelvins -
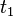 to
to 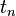 are time intervals at each of the sample points
are time intervals at each of the sample points
When the temperature readings are taken at the same interval (i.e., 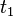 =
= 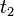 =
=  =
= 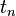 ), the above equation is reduced to:
), the above equation is reduced to:
Where:
-
 is the number of temperature sample points
is the number of temperature sample points
External links
| Wikimedia Commons has media related to Mean kinetic temperature. |
This article is issued from Wikipedia - version of the Monday, August 10, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.