Magnetic refrigeration

Magnetic refrigeration is a cooling technology based on the magnetocaloric effect. This technique can be used to attain extremely low temperatures, as well as the ranges used in common refrigerators. Compared to traditional gas-compression refrigeration, magnetic refrigeration is safer, quieter, more compact, has a higher cooling efficiency, and is more environmentally friendly because it does not use harmful, ozone-depleting coolant gases.[1][2][3]
The effect was first observed by French physicist P. Weiss and Swiss physicist A. Piccard in 1917.[4] The fundamental principle was suggested by P. Debye (1926) and W. Giauque (1927).[5] The first working magnetic refrigerators were constructed by several groups beginning in 1933. Magnetic refrigeration was the first method developed for cooling below about 0.3K (a temperature attainable by 3
He refrigeration, that is pumping on the 3
He vapors).
The magnetocaloric effect
The magnetocaloric effect (MCE, from magnet and calorie) is a magneto-thermodynamic phenomenon in which a temperature change of a suitable material is caused by exposing the material to a changing magnetic field. This is also known by low temperature physicists as adiabatic demagnetization. In that part of the refrigeration process, a decrease in the strength of an externally applied magnetic field allows the magnetic domains of a magnetocaloric material to become disoriented from the magnetic field by the agitating action of the thermal energy (phonons) present in the material. If the material is isolated so that no energy is allowed to (re)migrate into the material during this time, (i.e., an adiabatic process) the temperature drops as the domains absorb the thermal energy to perform their reorientation. The randomization of the domains occurs in a similar fashion to the randomization at the curie temperature of a ferromagnetic material, except that magnetic dipoles overcome a decreasing external magnetic field while energy remains constant, instead of magnetic domains being disrupted from internal ferromagnetism as energy is added.
One of the most notable examples of the magnetocaloric effect is in the chemical element gadolinium and some of its alloys. Gadolinium's temperature increases when it enters certain magnetic fields. When it leaves the magnetic field, the temperature drops. The effect is considerably stronger for the gadolinium alloy (Gd
5Si
2Ge
2).[6] Praseodymium alloyed with nickel (PrNi
5) has such a strong magnetocaloric effect that it has allowed scientists to approach to within one milliKelvin, one thousandth of a degree of absolute zero.[7]
Equation
The magnetocaloric effect can be quantified with the equation below:
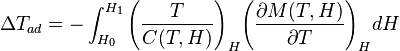
where T is the temperature, H is the applied magnetic field, C is the heat capacity of the working magnet (refrigerant) and M is the magnetization of the refrigerant.
From the equation we can see that magnetocaloric effect can be enhanced by:
- applying a large field
- using a magnet with a small heat capacity
- using a magnet with a large change in magnetization vs. temperature, at a constant magnetic field
Thermodynamic cycle

The cycle is performed as a refrigeration cycle that is analogous to the Carnot refrigeration cycle, but with increases and decreases in magnetic field strength instead of increases and decreases in pressure. It can be described at a starting point whereby the chosen working substance is introduced into a magnetic field, i.e., the magnetic flux density is increased. The working material is the refrigerant, and starts in thermal equilibrium with the refrigerated environment.
- Adiabatic magnetization: A magnetocaloric substance is placed in an insulated environment. The increasing external magnetic field (+H) causes the magnetic dipoles of the atoms to align, thereby decreasing the material's magnetic entropy and heat capacity. Since overall energy is not lost (yet) and therefore total entropy is not reduced (according to thermodynamic laws), the net result is that the substance is heated (T + ΔTad).
- Isomagnetic enthalpic transfer: This added heat can then be removed (-Q) by a fluid or gas — gaseous or liquid helium, for example. The magnetic field is held constant to prevent the dipoles from reabsorbing the heat. Once sufficiently cooled, the magnetocaloric substance and the coolant are separated (H=0).
- Adiabatic demagnetization: The substance is returned to another adiabatic (insulated) condition so the total entropy remains constant. However, this time the magnetic field is decreased, the thermal energy causes the magnetic moments to overcome the field, and thus the sample cools, i.e., an adiabatic temperature change. Energy (and entropy) transfers from thermal entropy to magnetic entropy (disorder of the magnetic dipoles).
- Isomagnetic entropic transfer: The magnetic field is held constant to prevent the material from reheating. The material is placed in thermal contact with the environment to be refrigerated. Because the working material is cooler than the refrigerated environment (by design), heat energy migrates into the working material (+Q).
Once the refrigerant and refrigerated environment are in thermal equilibrium, the cycle can restart.
Applied technique
The basic operating principle of an adiabatic demagnetization refrigerator (ADR) is the use of a strong magnetic field to control the entropy of a sample of material, often called the "refrigerant". Magnetic field constrains the orientation of magnetic dipoles in the refrigerant. The stronger the magnetic field, the more aligned the dipoles are, corresponding to lower entropy and heat capacity because the material has (effectively) lost some of its internal degrees of freedom. If the refrigerant is kept at a constant temperature through thermal contact with a heat sink (usually liquid helium) while the magnetic field is switched on, the refrigerant must lose some energy because it is equilibrated with the heat sink. When the magnetic field is subsequently switched off, the heat capacity of the refrigerant rises again because the degrees of freedom associated with orientation of the dipoles are once again liberated, pulling their share of equipartitioned energy from the motion of the molecules, thereby lowering the overall temperature of a system with decreased energy. Since the system is now insulated when the magnetic field is switched off, the process is adiabatic, i.e., the system can no longer exchange energy with its surroundings (the heat sink), and its temperature decreases below its initial value, that of the heat sink.
The operation of a standard ADR proceeds roughly as follows. First, a strong magnetic field is applied to the refrigerant, forcing its various magnetic dipoles to align and putting these degrees of freedom of the refrigerant into a state of lowered entropy. The heat sink then absorbs the heat released by the refrigerant due to its loss of entropy. Thermal contact with the heat sink is then broken so that the system is insulated, and the magnetic field is switched off, increasing the heat capacity of the refrigerant, thus decreasing its temperature below the temperature of the heat sink. In practice, the magnetic field is decreased slowly in order to provide continuous cooling and keep the sample at an approximately constant low temperature. Once the field falls to zero or to some low limiting value determined by the properties of the refrigerant, the cooling power of the ADR vanishes, and heat leaks will cause the refrigerant to warm up.
Working materials
The magnetocaloric effect (MCE) is an intrinsic property of a magnetic solid. This thermal response of a solid to the application or removal of magnetic fields is maximized when the solid is near its magnetic ordering temperature. Thus, the materials considered for magnetic refrigeration devices should be magnetic materials with a magnetic phase transition temperature near the temperature region of interest.[8] For refrigerators that could be used in the home, this temperature is room temperature. The temperature change can be further increased when the order-parameter of the phase transition changes strongly within the temperature range of interest.[1]
The magnitudes of the magnetic entropy and the adiabatic temperature changes are strongly dependent upon the magnetic ordering process. The magnitude is generally small in antiferromagnets, ferrimagnets and spin glass systems but can be much larger for ferromagnets that undergo a magnetic phase transition. First order phase transitions are characterized by a discontinuity in the magnetization changes with temperature, resulting in a latent heat.[8] Second order phase transitions do not have this latent heat associated with the phase transition.[8]
In the late 1990s Pecharksy and Gschneidner reported a magnetic entropy change in Gd
5(Si
2Ge
2) that was about 50% larger than that reported for Gd metal, which had the largest known magnetic entropy change at the time.[9] This giant magnetocaloric effect (GMCE) occurred at 270K, which is lower than that of Gd (294K).[3] Since the MCE occurs below room temperature these materials would not be suitable for refrigerators operating at room temperature.[10] Since then other alloys have also demonstrated the giant magnetocaloric effect. These include Gd
5(Si
xGe
1−x)
4, La(Fe
xSi
1−x)
13H
x and MnFeP
1−xAs
x alloys,.[8][10] Gadolinium and its alloys undergo second-order phase transitions that have no magnetic or thermal hysteresis.[11] However, the use of rare earth elements makes these materials very expensive.
Ni
2Mn-X (X = Ga, Co, In, Al, Sb) Heusler alloys are also promising candidates for magnetic cooling applications because they have Curie temperatures near room temperature and, depending on composition, can have martensitic phase transformations near room temperature.[2] These materials exhibit the magnetic shape memory effect and can also be used as actuators, energy harvesting devices, and sensors.[12] When the martensitic transformation temperature and the Curie temperature are the same (based on composition) the magnitude of the magnetic entropy change is the largest.[1] In February 2014, GE announced the development of a functional Ni-Mn-based magnetic refrigerator.[13][14]
The development of this technology is very material-dependent and will likely not replace vapor-compression refrigeration without significantly improved materials that are cheap, abundant, and exhibit much larger magnetocaloric effects over a larger range of temperatures. Such materials need to show significant temperature changes under a field of two tesla or less, so that permanent magnets can be used for the production of the magnetic field.[15][16]
Paramagnetic salts
The original proposed refrigerant was a paramagnetic salt, such as cerium magnesium nitrate. The active magnetic dipoles in this case are those of the electron shells of the paramagnetic atoms.
In a paramagnetic salt ADR, the heat sink is usually provided by a pumped 4
He (about 1.2 K) or 3
He (about 0.3 K) cryostat. An easily attainable 1 T magnetic field is generally required for initial magnetization. The minimum temperature attainable is determined by the self-magnetization tendencies of the refrigerant salt, but temperatures from 1 to 100 mK are accessible. Dilution refrigerators had for many years supplanted paramagnetic salt ADRs, but interest in space-based and simple to use lab-ADRs has remained, due to the complexity and unreliability of the dilution refrigerator
Eventually paramagnetic salts become either diamagnetic or ferromagnetic, limiting the lowest temperature that can be reached using this method.
Nuclear demagnetization
One variant of adiabatic demagnetization that continues to find substantial research application is nuclear demagnetization refrigeration (NDR). NDR follows the same principles, but in this case the cooling power arises from the magnetic dipoles of the nuclei of the refrigerant atoms, rather than their electron configurations. Since these dipoles are of much smaller magnitude, they are less prone to self-alignment and have lower intrinsic minimum fields. This allows NDR to cool the nuclear spin system to very low temperatures, often 1 µK or below. Unfortunately, the small magnitudes of nuclear magnetic dipoles also makes them less inclined to align to external fields. Magnetic fields of 3 teslas or greater are often needed for the initial magnetization step of NDR.
In NDR systems, the initial heat sink must sit at very low temperatures (10–100 mK). This precooling is often provided by the mixing chamber of a dilution refrigerator or a paramagnetic salt.
Commercial development
Research and a demonstration proof of concept in 2001 succeeded in applying commercial-grade materials and permanent magnets at room temperatures to construct a magnetocaloric refrigerator[17]
On August 20, 2007, the Risø National Laboratory (Denmark) at the Technical University of Denmark, claimed to have reached a milestone in their magnetic cooling research when they reported a temperature span of 8.7 K.[18] They hope to introduce the first commercial applications of the technology by 2010.
As of 2013 this technology had proven commercially viable only for ultra-low temperature cryogenic applications available for decades. Magnetocaloric refrigeration systems are composed of pumps, motors, secondary fluids, heat exchangers of different types, magnets and magnetic materials. These processes are greatly affected by irreversibilities and should be adequately considered. At year-end, Cooltech Applications[19] announced that its first commercial refrigeration equipment would enter the market in 2014. At the 2015 Consumer Electronics Show in Las Vegas, a consortium of Haier, Astronautics Corporation of America and BASF presented the first cooling appliance.[20] BASF claim of their technology a 35% improvement over using compressors[21]
Current and future uses
Thermal and magnetic hysteresis problems remain to be solved for first-order phase transition materials that exhibit the GMCE.[15]
One potential application is in spacecraft.
Vapor-compression refrigeration units typically achieve performance coefficients of 60% of that of a theoretical ideal Carnot cycle, much higher than current MR technology. Small domestic refrigerators are however much less efficient.[22]
In 2014 giant anisotropic behaviour of the magnetocaloric effect was found in HoMn
2O
5 at 10 K. The anisotropy of the
magnetic entropy change gives rise to a large rotating MCE offering the possibility to build simplified, compact, and efficient magnetic cooling systems by rotating it in a constant magnetic field.[23]
History
The effect was discovered using nickel in 1917 by French physicist Pierre Weiss and Auguste Piccard.[24] Originally, the cooling effect was less than 0.5 K/T.
Major advances first appeared in the late 1920s when cooling via adiabatic demagnetization was independently proposed by Peter Debye in 1926 and chemistry Nobel Laureate William F. Giauque in 1927.
It was first demonstrated experimentally by Giauque and his colleague D. P. MacDougall in 1933 for cryogenic purposes when they reached 0.25 K.[25] Between 1933 and 1997, advances in MCE cooling occurred.[26]
In 1997, the first near room-temperature proof of concept magnetic refrigerator was demonstrated by Karl A. Gschneidner, Jr. by the Iowa State University at Ames Laboratory. This event attracted interest from scientists and companies worldwide who started developing new kinds of room temperature materials and magnetic refrigerator designs.[6]
A major breakthrough came 2002 when a group at the University of Amsterdam demonstrated the giant magnetocaloric effect in MnFe(P,As) alloys that are based on abundant materials.[27]
Refrigerators based on the magnetocaloric effect have been demonstrated in laboratories, using magnetic fields starting at 0.6 T up to 10 T. Magnetic fields above 2 T are difficult to produce with permanent magnets and are produced by a superconducting magnet (1 T is about 20,000 times the Earth's magnetic field).
Room temperature devices
Recent research has focused on near room temperature. Constructed examples of room temperature magnetic refrigerators include:
| Sponsor | Location | Announcement date | Type | Max. cooling power (W)[1] | Max ΔT (K)[2] | Magnetic field (T) | Solid refrigerant | Quantity (kg) |
|---|---|---|---|---|---|---|---|---|
| Ames Laboratory/Astronautics[28] | Ames, Iowa/Madison, Wisconsin, USA | February 20, 1997 | Reciprocating | 600 | 10 | 5 (S) | Gd spheres | |
| Mater. Science Institute Barcelona[29] | Barcelona, Spain | May 2000 | Rotary | ? | 5 | 0.95 (P) | Gd foil | |
| Chubu Electric/Toshiba[30] | Yokohama, Japan | Summer 2000 | Reciprocating | 100 | 21 | 4 (S) | Gd spheres | |
| University of Victoria[31][32] | Victoria, British Columbia Canada | July 2001 | Reciprocating | 2 | 14 | 2 (S) | Gd & Gd 1−xTb x L.B. | |
| Astronautics[33] | Madison, Wisconsin, USA | September 18, 2001 | Rotary | 95 | 25 | 1.5 (P) | Gd spheres | |
| Sichuan Inst. Tech./Nanjing University[34] | Nanjing, China | 23 April 2002 | Reciprocating | ? | 23 | 1.4 (P) | Gd spheres and Gd5Si1.985Ge1.985Ga0.03 powder | |
| Chubu Electric/Toshiba[35] | Yokohama, Japan | October 5, 2002 | Reciprocating | 40 | 27 | 0.6 (P) | Gd 1−xDy x L.B. | |
| Chubu Electric/Toshiba[35] | Yokohama, Japan | March 4, 2003 | Rotary | 60 | 10 | 0.76 (P) | Gd 1−xDy x L.B. | 1 |
| Lab. d’Electrotechnique Grenoble[36] | Grenoble, France | April 2003 | Reciprocating | 8.8 | 4 | 0.8 (P) | Gd foil | |
| George Washington University [37] | USA | July 2004 | Reciprocating | ? | 5 | 2 (P) | Gd foil | |
| Astronautics[38] | Madison, Wisconsin, USA | 2004 | Rotary | 95 | 25 | 1.5 (P) | Gd and GdEr spheres / La(Fe 0.88Si130− 0.12H 1.0 | |
| University of Victoria[39] | Victoria, British Columbia Canada | 2006 | Reciprocating | 15 | 50 | 2 (S) | Gd, Gd 0.74Tb 0.26 and Gd 0.85Er 0.15 pucks | 0.12 |
| 1maximum cooling power at zero temperature difference (ΔT=0); 2maximum temperature span at zero cooling capacity (W=0); L.B. = layered bed; P = permanent magnet; S = superconducting magnet | ||||||||
In one example, Prof. Karl A. Gschneidner, Jr. unveiled a proof of concept magnetic refrigerator near room temperature on February 20, 1997. He also announced the discovery of the GMCE in Gd
5Si
2Ge
2 on June 9, 1997.[9] Since then, hundreds of peer-reviewed articles have been written describing materials exhibiting magnetocaloric effects.
See also
References
- 1 2 3 Brück, E. (2005). "Developments in magnetocaloric refrigeration". Journal of Physics D: Applied Physics 38 (23): R381. doi:10.1088/0022-3727/38/23/R01.
- 1 2 Khovaylo, V. V.; Rodionova, V. V.; Shevyrtalov, S. N.; Novosad, V. (2014). "Magnetocaloric effect in "reduced" dimensions: Thin films, ribbons, and microwires of Heusler alloys and related compounds". Physica status solidi (b) 251 (10): 2104. doi:10.1002/pssb.201451217.
- 1 2 Gschneidner, K. A.; Pecharsky, V. K. (2008). "Thirty years of near room temperature magnetic cooling: Where we are today and future prospects". International Journal of Refrigeration 31 (6): 945. doi:10.1016/j.ijrefrig.2008.01.004.
- ↑ Weiss, Pierre; Piccard, Auguste (1917). "Le phénomène magnétocalorique". J. Phys. (Paris). 5th Ser. (7): 103–109.
Smith, Anders (2013). "Who discovered the magnetocaloric effect?". The European Physical Journal H 38 (4): 507–517. Bibcode:2013EPJH...38..507S. doi:10.1140/epjh/e2013-40001-9. - ↑ Zemansky, Mark W. (1981). Temperatures very low and very high. New York: Dover. p. 50. ISBN 0-486-24072-X.
- 1 2 Karl Gschneidner, Jr. & Kerry Gibson (December 7, 2001). "Magnetic Refrigerator Successfully Tested". Ames Laboratory News Release. Ames Laboratory. Retrieved 2006-12-17.
- ↑ Emsley, John (2001). Nature's Building Blocks. Oxford University Press. p. 342. ISBN 0-19-850341-5.
- 1 2 3 4 Smith, A.; Bahl, C. R. H.; Bjørk, R.; Engelbrecht, K.; Nielsen, K. K.; Pryds, N. (2012). "Materials Challenges for High Performance Magnetocaloric Refrigeration Devices". Advanced Energy Materials 2 (11): 1288. doi:10.1002/aenm.201200167.
- 1 2 Pecharsky, V. K.; Gschneidner, Jr., K. A. (1997). "Giant Magnetocaloric Effect in Gd_{5}(Si_{2}Ge_{2})". Physical Review Letters 78 (23): 4494. doi:10.1103/PhysRevLett.78.4494.
- 1 2 Moya, X.; Kar-Narayan, S.; Mathur, N. D. (2014). "Caloric materials near ferroic phase transitions". Nature Materials 13 (5): 439. doi:10.1038/NMAT3951.
- ↑ Song, N. N.; Ke, Y. J.; Yang, H. T.; Zhang, H.; Zhang, X. Q.; Shen, B. G.; Cheng, Z. H. (2013). "Integrating giant microwave absorption with magnetic refrigeration in one multifunctional intermetallic compound of LaFe11.6Si1.4C0.2H1.7". Scientific Reports 3. doi:10.1038/srep02291.
- ↑ Dunand, D. C.; Müllner, P. (2011). "Size Effects on Magnetic Actuation in Ni-Mn-Ga Shape-Memory Alloys". Advanced Materials 23 (2): 216. doi:10.1002/adma.201002753. PMID 20957766.
- ↑ "GE Global Research Live".
- ↑ "Your next fridge could keep cold more efficiently using magnets". gizmag.com.
- 1 2 Gschneidnerjr, K. A.; Pecharsky, V. K.; Tsokol, A. O. (2005). "Recent developments in magnetocaloric materials". Reports on Progress in Physics 68 (6): 1479. doi:10.1088/0034-4885/68/6/R04.
- ↑ Pecharsky, V. K.; Gschneidner Jr, K. A. (1999). "Magnetocaloric effect and magnetic refrigeration". Journal of Magnetism and Magnetic Materials 200: 44. doi:10.1016/S0304-8853(99)00397-2.
- ↑ Gibson, Kerry (November 2001). "Magnetic Refrigerator Successfully Tested: Ames Laboratory developments help push boundaries of new refrigeration technology". INSIDER Newsletter for employees of Ames Laboratory.(Vol. 112, No.10 )
- ↑ Milestone in magnetic cooling, Risø News, August 20, 2007. Retrieved August 28, 2007.
- ↑ "Cooltech Applications". Cooltech Applications. Retrieved 2014-06-04.
- ↑ "Premiere of cutting-edge magnetocaloric cooling appliance". BASF. Retrieved 16 July 2015.
- ↑ http://www.basf-new-business.com/en/projects/e-power-management/solid-state-cooling/
- ↑ "Information Bridge: DOE Scientific and Technical Information - Sponsored by OSTI" (PDF). Osti.gov. 2012-08-31. Retrieved 2012-10-04.
- ↑ Balli, M.; Jandl, S.; Fournier, P.; Gospodinov, M. M. (2014). "Anisotropy-enhanced giant reversible rotating magnetocaloric effect in HoMn2O5 single crystals" (PDF). Applied Physics Letters 104 (6868): 232402–1 to 5. Bibcode:2014ApPhL.104w2402B. doi:10.1063/1.4880818.
- ↑ Weiss, Pierre; Piccard, Auguste (1917). "Le phenomene magnetocalorique.". J. Phys. Theor. Appl. 7 (1): 103–109. doi:10.1051/jphystap:019170070010300.
- ↑ Giauque, W. F.; MacDougall, D. P. (1933). "Attainment of Temperatures Below 1° Absolute by Demagnetization of Gd2(SO4)3·8H2O". Phys. Rev. 43 (9): 768. Bibcode:1933PhRv...43..768G. doi:10.1103/PhysRev.43.768.
- ↑ Gschneidner, K. A. Jr.; Pecharsky, V. K. (1997). Bautista, R. G.; et al., eds. Rare Earths: Science, Technology and Applications III. Warrendale, PA: The Minerals, Metals and Materials Society. p. 209.
Pecharsky, V. K.; Gschneidner, K. A. Jr. (1999). "Magnetocaloric Effect and Magnetic Refrigeration". J. Magn. Magn. Mater. 200 (1–3): 44–56. Bibcode:1999JMMM..200...44P. doi:10.1016/S0304-8853(99)00397-2.
Gschneidner, K. A. Jr.; Pecharsky, V. K. (2000). "Magnetocaloric Materials". Annu. Rev. Mater. Sci. 30 (1): 387–429. Bibcode:2000AnRMS..30..387G. doi:10.1146/annurev.matsci.30.1.387.
Gschneidner, K. A. Jr.; Pecharsky, V. K. (2002). Chandra, D.; Bautista, R. G., eds. Fundamentals of Advanced Materials for Energy Conversion. Warrendale, PA: The Minerals, Metals and Materials Society. p. 9. - ↑ Tegus, O.; Brück, E.; de Boer, F. R.; Buschow, K. H. J. (2002). "Transition-metal-based magnetic refrigerants for room-temperature applications". Nature 415 (6868): 150–152. Bibcode:2002Natur.415..150T. doi:10.1038/415150a.
- ↑ Zimm, C; Jastrab, A.; Sternberg, A.; Pecharsky, V.K.; Gschneidner, K.A. Jr.; Osborne, M.; Anderson, I. (1998). Adv. Cryog. Eng. 43: 1759. Missing or empty
|title=(help) - ↑ Lee, S. J.; Kenkel, J. M.; Pecharsky, V. K.; Jiles, D. C. (2002). "Permanent magnet array for the magnetic refrigerator". Journal of Applied Physics 91 (10): 8894. doi:10.1063/1.1451906.
- ↑ Hirano, N. (2002). "Development of magnetic refrigerator for room temperature application". AIP Conference Proceedings 613. p. 1027. doi:10.1063/1.1472125.
- ↑ Rowe A.M. and Barclay J.A., Adv. Cryog. Eng. 47 995 (2002).
- ↑ Richard, M. -A. (2004). "Magnetic refrigeration: Single and multimaterial active magnetic regenerator experiments". Journal of Applied Physics 95 (4): 2146. doi:10.1063/1.1643200.
- ↑ Zimm C, Paper No K7.003 Am. Phys. Soc. Meeting, March 4, Austin, Texas (2003)
- ↑ Wu W., Paper No. K7.004 Am. Phys. Soc. Meeting, March 4, Austin, Texas (2003)
- 1 2 Hirano N., Paper No. K7.002 Am. Phys. Soc. Meeting March 4, Austin, Texas,
- ↑ Clot, P.; Viallet, D.; Allab, F.; Kedous-Lebouc, A.; Fournier, J. M.; Yonnet, J. P. (2003). "A magnet-based device for active magnetic regenerative refrigeration". IEEE Transactions on Magnetics 39 (5): 3349. doi:10.1109/TMAG.2003.816253.
- ↑ Shir, F.; Mavriplis, C.; Bennett, L. H.; Torre, E. D. (2005). "Analysis of room temperature magnetic regenerative refrigeration". International Journal of Refrigeration 28 (4): 616. doi:10.1016/j.ijrefrig.2004.08.015.
- ↑ Zimm C, Paper No. K7.003 Am. Phys. Soc. Meeting, March 4, Austin, Texas (2003)
- ↑ Rowe, A.; Tura, A. (2006). "Experimental investigation of a three-material layered active magnetic regenerator". International Journal of Refrigeration 29 (8): 1286. doi:10.1016/j.ijrefrig.2006.07.012.
Further reading
- Lounasmaa, Experimental Principles and Methods Below 1 K, Academic Press (1974).
- Richardson and Smith, Experimental Techniques in Condensed Matter Physics at Low Temperatures, Addison Wesley (1988).
- Lucia, U. General approach to obtain the magnetic refrigeretion ideal Coefficient of Performance COP, Physica A: Statistical Mechanics and its Applications, 387/14 (2008) 3477–3479, doi:10.1016/j.physa.2008.02.026; see also http://arxiv.org/abs/1011.1684
External links
- NASA – How does an Adiabatic Demagnetization Refrigerator Work ?
- What is magnetocaloric effect and what materials exhibit this effect the most?
- Magnetocaloric materials keep fridges cool by C. Wu
- Ames Laboratory news release, May 25, 1999, Work begins on prototype magnetic-refrigeration unit.
- Magnetic refrigerator successfully tested
- Refrigeration Systems Terry Heppenstall's notes, University of Newcastle upon Tyne (November 2000)
- XRS Adiabatic Demagnetization Refrigerator
- Executive Summary: A Continuous Adiabatic Demagnetization Refrigerator (.doc format) (Google cache)
- Origin and tuning of the magnetocaloric effect in the magnetic refrigerant Mn1.1Fe0.9(P0.8Ge0.2)
- Magnetic technology revolutionizes refrigeration
- Evaluation of thermodynamic quantities in magnetic refrigeration
- All About Magnetic Refrigeration - SIRACH
|