Magnetic chemistry

Magnetic chemistry are chemical reactions in which either reactant, reagent or product have magnetic properties. Even though this definition in theory includes even single magnetic atoms, in practice the smallest magnetic units are magnetic nanoparticles. Magnetic chemistry and its applications are an interdisciplinary field between chemistry, biology, material sciences and chemical engineering.[2][3]
Field of use
Since magnetic nanoparticles are time and cost intensive to produce, their use only makes sense in reactions where they can be either reused, used only in catalytic amounts, or the resulting product is even more precious. So far, magnetic nanoparticles are used in connection with magnetic chemistry only in scientific research such as a biocatalyst,[4] catalyst, catalyst support[3] and solid phase synthesis support,[5] as well in biotechnology[6] and medicine.[7] An industrial use has yet to be established. The potential and versatility of magnetic chemistry arises from the fast and easy separation of the magnetic nanoparticles, eliminating tedious and costly separation processes usually applied in chemistry. Furthermore the magnetic nanoparticles can be guided via a magnetic field to the desired location which could, for example, enable pinpoint precision in fighting cancer.
Biotechnology and medical applications
Enzymes, proteins and other biologically and chemically active substances have been immobilized on magnetic nanoparticles.[4] Allowing for reactions even within the human body itself such as cellular labelling/cell separation, detoxification of biological fluids, tissue repair, drug delivery, magnetic resonance imaging, hyperthermia and magnetofection.[7]
Direct catalysis
Uncoated metallic magnetic nanoparticles are very prone to oxidation which makes them unsuitable for direct applications in catalysis.
Catalyst support
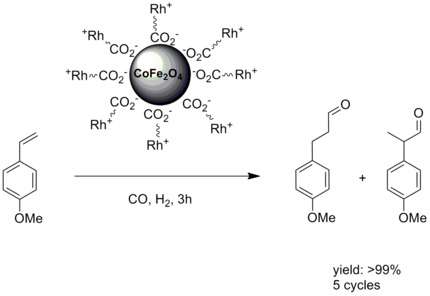
Conventional solid support catalysis often suffers from reduced reactivity and selectivity due to the fact that the catalytic active center is embedded in a solid support.[3] Immobilizing the catalytic center on top of nanoparticles with a large surface to volume ratio counters this problem. In the case of magnetic nanoparticles it adds the property of facile a separation. An early example of a catalysis with Rhodium attached to magnetic nanoparticles was shown by T.-J Yoon et al.[8]
An other example of a catalyst immobilized onto magnetic nanoparticles is shown by Schätz et al..[9] The catalyst in this case is the stable radical TEMPO, which was attached to the graphene coated Cobalt nanoparticles in several steps starting with a diazonium reaction, known well from carbon nanotubes, and successive click chemistry. The resulting catalyst was then successfully used for the chemoselective oxidation of primary and secondary alcohols.
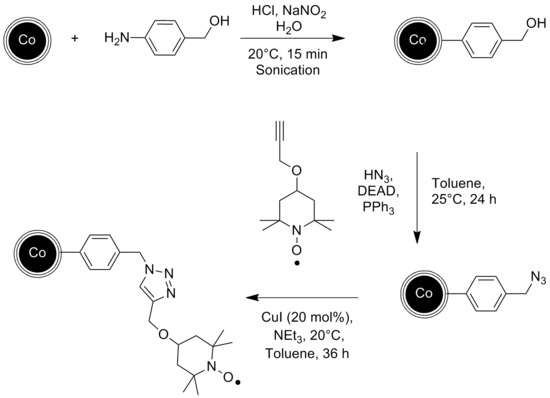
Thanks to the magnetic properties of the nanoparticles, the catalytic reaction can take place in a continuous flow reactor instead of a batch reactor with no remains of the catalyst in the end product. Graphene coated cobalt nanoparticles have been used for that experiment since they exhibit a higher magnetization than Ferrite nanoparticles, which is essential for a fast and clean separation via external magnetic field.[10]

Solid support synthesis
Various molecular structures have been synthesized on the surface of modified magnetic nanoparticles, for example peptides.[5]
References
- ↑ Grass, R. N.; Athanassiou, E. K.; Stark, W. J. (2007). "Covalently Functionalized Cobalt Nanoparticles as a Platform for Magnetic Separations in Organic Synthesis". Angewandte Chemie International Edition 46 (26): 4909. doi:10.1002/anie.200700613.
- ↑ S. B. Darling, S. B.; S. D. Bader (2005). "A materials chemistry perspective on nanomagnetism". Journal of Materials Chemistry 15 (39): 4189–4195. doi:10.1039/B506357D.
- 1 2 3 A. Schätz, Alexander; O. Reiser; W.J. Stark (2010). "Nanoparticles as Semi-Heterogeneous Catalyst Supports". Chem. Eur. J. 16 (30): 8950–67. doi:10.1002/chem.200903462.
- 1 2 Huang-Hao Yang, Huang-Hao; Shu-Qiong Zhang; Xiao-Lan Chen; Zhi-Xia Zhuang; Jin-Gou Xu; Xiao-Ru Wang (2004). "Magnetite-Containing Spherical Silica Nanoparticles for Biocatalysis and Bioseparations". Analytical Chemistry 76 (5): 1316–1321. doi:10.1021/ac034920m. PMID 14987087.
- 1 2 K.Norén, Katarina; M. Kempe (2009). "Multilayered Magnetic Nanoparticles as a Support in Solid-Phase Peptide Synthesis". International Journal of Peptide Research and Therapeutics 15 (4): 287–292. doi:10.1007/s10989-009-9190-3.
- ↑ An-Hui Lu, An-Hui; E. L. Salabas; Ferdi Schüth (2007). "Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application". Angewandte Chemie International Edition 46 (8): 1222–1244. doi:10.1002/anie.200602866.
- 1 2 Gupta AK, Ajay Kumar; Gupta M (2005). "Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications". Biomaterials 26 (18): 3995–4021. doi:10.1016/j.biomaterials.2004.10.012. PMID 15626447.
- ↑ Tae-Jong Yoon, Tae-Jong; Woo Lee; Yoon-Seuk Oh; Jin-Kyu Lee (2003). "Magnetic nanoparticles as a catalyst vehicle for simple and easy recyclingElectronic supplementary information (ESI) available: XRD and FT-IR data, as well as the detailed experimental conditions for the catalytic hydroformylation reactions. See http://www.rsc.org/suppdata/nj/b2/b209391j/". New Journal of Chemistry 27 (2): 227.229. doi:10.1039/B209391J. External link in
|title=(help) - ↑ A. Schätz, Alexander; R. N. Grass; W. J. Stark; O. Reiser (2008). "TEMPO Supported on Magnetic C/Co-Nanoparticles: A Highly Active and Recyclable Organocatalyst". Chemistry - A European Journal 14 (27): 8262–8266. doi:10.1002/chem.200801001.
- ↑ A. Schätz, Alexander; R. N. Grass; Q. Kainz; W. J. Stark; O. Reiser (2010). "Cu(II)−Azabis(oxazoline) Complexes Immobilized on Magnetic Co/C Nanoparticles: Kinetic Resolution of 1,2-Diphenylethane-1,2-diol under Batch and Continuous-Flow Conditions". Chemistry of Materials 22 (2): 305–310. doi:10.1021/cm9019099.