Monoamine oxidase B
Monoamine oxidase B, also known as MAOB, is an enzyme that in humans is encoded by the MAOB gene.
The protein encoded by this gene belongs to the flavin monoamine oxidase family. It is an enzyme located in the outer mitochondrial membrane. It catalyzes the oxidative deamination of biogenic and xenobiotic amines and plays an important role in the catabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues. This protein preferentially degrades benzylamine and phenylethylamine.[1] Like MAOA, it also degrades dopamine.
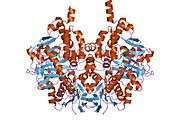
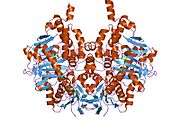
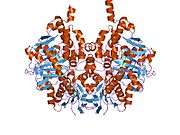
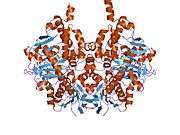
Structure
Monoamine oxidase B has a hydrophobic bipartite elongated cavity that (for the "open" conformation) occupies a combined volume close to 700 Å3. hMAO-A has a single cavity that exhibits a rounder shape and is larger in volume than the "substrate cavity" of hMAO-B.[2]
The first cavity of hMAO-B has been termed the entrance cavity (290 Å3), the second substrate cavity or active site cavity (~390 Å3) – between both an isoleucine199 side-chain serves as a gate. Depending on the substrate or bound inhibitor, it can exist in either an open or a closed form, which has been shown to be important in defining the inhibitor specificity of hMAO B. At the end of the substrate cavity is the FAD coenzyme with sites for favorable amine binding about the flavin involving two nearly parallel tyrosyl (398 and 435) residues that form what has been termed an aromatic cage.[2]
Differences between MAOA and MAOB
MAO-A is involved in the metabolism of tyramine; inhibition, in particular irreversible inhibition of MAO-A can result in a dangerous pressor effect when foods high in tyramine are consumed such as cheeses. MAO-A is involved in the metabolism of serotonin, noradrenaline and dopamine whereas MAO-B metabolises the dopamine neurotransmitter.[3] MAO-B is an enzyme on the outer mitochondrial membrane and catalyzes the oxidation of arylalkylamine neurotransmitters[4]
Monoamine oxidase A (MAOA) generally metabolizes tyramine, norepinephrine (NE), serotonin (5-HT), and dopamine (DA) (and other less clinically relevant chemicals). In contrast, Monoamine oxidase B (MAOB) mainly metabolizes dopamine (DA) (and other less clinically relevant chemicals). The differences between the substrate selectivity of the two enzymes are utilized clinically when treating specific disorders: Monoamine oxidase A inhibitors have been typically used in the treatment of depression, and monoamine oxidase B inhibitors are typically used in the treatment of Parkinson's disease.[5][6] Nonspecific (i.e. MAOA/B combined) inhibitors can pose problems when taken concomitantly with tyramine-containing foods such as cheese, because the drug's inhibition of MAOA causes a dangerous elevation of serum tyramine levels, which can lead to hypertensive symptoms. Selective MAOB inhibitors bypass this problem by preferentially inhibiting MAOB, which mostly metabolizes DA. If MAOB is inhibited, then more DA is available for proper neuronal function, especially in Parkinson's Disease.
Roles in disease and aging
Alzheimer's disease and Parkinson's disease are both associated with elevated levels of MAO-B in the brain.[7][8] The normal activity of MAO-B creates reactive oxygen species, which directly damage cells.[9] MAO-B levels have been found to increase with age, suggesting a role in natural age related cognitive decline and the increased likelihood of developing neurological diseases later in life.[10] More active polymorphisms of the MAOB gene have been linked to negative emotionality, and suspected as an underlying factor in depression.[11] Activity of MAO-B has also been shown to play a role in stress-induced cardiac damage.[12][13]
Animal models
Transgenic mice that are unable to produce MAO-B are shown to be resistant to a mouse model of Parkinson's disease.[14][15][16] They also demonstrate increased responsiveness to stress (as with MAO-A knockout mice)[17] and increased β-PEA.[15][17] In addition, they exhibit behavioral disinhibition and reduced anxiety-like behaviors.[18]
Inhibition of MAO-B in rats has been shown to prevent many age-related biological changes such as optic nerve degeneration, and extend average lifespan by up to 39%.[19][20]
Effects of deficiency in humans
While people lacking the gene for MAO-A display mental retardation and behavioral abnormalities, people lacking the gene for MAO-B display no abnormalities except elevated phenethylamine levels in urine, raising the question of whether MAO-B is actually a necessary enzyme. Newer research indicates the importance of phenethylamine and other trace amines, which are now known to regulate catecholamine and serotonin neurotransmission through the same receptor as amphetamine, TAAR1.[21][22]
The prophylactic use of MAO-B inhibitors to slow natural human aging in otherwise healthy individuals has been proposed, but remains a highly controversial topic.[23][24]
Selective inhibitors

-Catechin.png)

Species-dependent divergences may hamper the extrapolation of inhibitor potencies.[25]
Reversible
Natural
- Geiparvarin[26]
- Desmethoxyyangonin,[27] a constituent of kava extract; modest affinity
- Catechin and epicatechin
- Garlic[28]
Synthetic
- Safinamide and analogs[29]
- 5H-Indeno[1,2-c]pyridazin-5-ones[25][30][31] (see 3d model)
- Substituted chalcones[32]
- 2-(N-Methyl-N-benzylaminomethyl)-1H-pyrrole[33]
- 1-(4-Arylthiazol-2-yl)-2-(3-methylcyclohexylidene)hydrazine[34]
- 2-Thiazolylhydrazone[35]
- 3,5-Diaryl pyrazole[36]
- Pyrazoline derivatives[37][38]
- Several coumarin derivatives[39] and #C19*[25] (see 3d model)
- Phenylcoumarins, extremely subtype selective[40] and further analogs[41][42][43] (see 3d model)
- Chromone-3-phenylcarboxamides[44]
- Isatins[45]
- Phthalimides[46]
- 8-Benzyloxycaffeines[47][48] and CSC analogs[49]
- (E,E)-8-(4-phenylbutadien-1-yl)caffeines,[50] with A2A antagonistic component
- Indazole- and Indole-5-carboxamides[51]
Irreversible (covalent)
- Selegiline (Eldepryl, Zelapar, Emsam)
- Rasagiline (Azilect)
References
- ↑ "Entrez Gene: MAOB monoamine oxidase B".
- 1 2 Edmondson DE, Binda C, Mattevi A (August 2007). "Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B". Arch. Biochem. Biophys. 464 (2): 269–76. doi:10.1016/j.abb.2007.05.006. PMC 1993809. PMID 17573034.
- ↑ Youdim MB, Weinstock M (January 2004). "Therapeutic applications of selective and non-selective inhibitors of monoamine oxidase A and B that do not cause significant tyramine potentiation". Neurotoxicology 25 (1–2): 243–50. doi:10.1016/S0161-813X(03)00103-7. PMID 14697899.
- ↑ Binda C, Hubálek F, Li M, Herzig Y, Sterling J, Edmondson DE, Mattevi A (March 2004). "Crystal structures of monoamine oxidase B in complex with four inhibitors of the N-propargylaminoindan class". J. Med. Chem. 47 (7): 1767–74. doi:10.1021/jm031087c. PMID 15027868.
- ↑ Nolen WA, Hoencamp E, Bouvy PF, Haffmans PM (1993). "Reversible monoamine oxidase-A inhibitors in resistant major depression". Clin Neuropharmacol 16 (Suppl 2): S69–76. PMID 8313400.
- ↑ Riederer P, Laux G (March 2011). "MAO-inhibitors in Parkinson's Disease". Exp Neurobiol 20 (1): 1–17. doi:10.5607/en.2011.20.1.1. PMC 3213739. PMID 22110357.
- ↑ Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G, Löffler J, Richards JG (September 1994). "Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography". Neuroscience 62 (1): 15–30. doi:10.1016/0306-4522(94)90311-5. PMID 7816197.
- ↑ Mallajosyula JK, Chinta SJ, Rajagopalan S, Nicholls DG, Andersen JK (October 2009). "Metabolic control analysis in a cellular model of elevated MAO-B: relevance to Parkinson's disease". Neurotox Res 16 (3): 186–93. doi:10.1007/s12640-009-9032-2. PMC 2727365. PMID 19526285.
- ↑ Nagatsu T, Sawada M (2006). "Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson's disease: possible implications of glial cells". J. Neural Transm. Suppl. Journal of Neural Transmission. Supplementa 71 (71): 53–65. doi:10.1007/978-3-211-33328-0_7. ISBN 978-3-211-33327-3. PMID 17447416.
- ↑ Kumar MJ, Andersen JK (August 2004). "Perspectives on MAO-B in aging and neurological disease: where do we go from here?". Mol. Neurobiol. 30 (1): 77–89. doi:10.1385/MN:30:1:077. PMID 15247489.
- ↑ Dlugos AM, Palmer AA, de Wit H (October 2009). "Negative emotionality: monoamine oxidase B gene variants modulate personality traits in healthy humans". J Neural Transm 116 (10): 1323–34. doi:10.1007/s00702-009-0281-2. PMC 3653168. PMID 19657584.
- ↑ Kaludercic N, Carpi A, Menabò R, Di Lisa F, Paolocci N (July 2011). "Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury". Biochim. Biophys. Acta 1813 (7): 1323–32. doi:10.1016/j.bbamcr.2010.09.010. PMC 3030628. PMID 20869994.
- ↑ Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, Paolocci N (January 2014). "Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts". Antioxid. Redox Signal. 20 (2): 267–80. doi:10.1089/ars.2012.4616. PMC 3887464. PMID 23581564.
- ↑ Shih JC, Chen K (1999). "MAO-A and -B gene knock-out mice exhibit distinctly different behavior". Neurobiology (Bp) 7 (2): 235–46. PMID 10591056.
- 1 2 Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, Karoum F, Gal J, Shih JC (October 1997). "Increased stress response and beta-phenylethylamine in MAOB-deficient mice.". Nature Genetics 17 (2): 206–10. doi:10.1038/ng1097-206. PMID 9326944.
- ↑ Shih JC, Chen K, Ridd MJ (1999). "Monoamine oxidase: from genes to behavior.". Annual review of neuroscience 22: 197–217. doi:10.1146/annurev.neuro.22.1.197. PMC 2844879. PMID 10202537.
- 1 2 Shih JC (January 2004). "Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B.". Neurotoxicology 25 (1–2): 21–30. doi:10.1016/s0161-813x(03)00112-8. PMID 14697877.
- ↑ Bortolato M, Godar SC, Davarian S, Chen K, Shih JC (December 2009). "Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice.". Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34 (13): 2746–57. doi:10.1038/npp.2009.118. PMC 2783894. PMID 19710633.
- ↑ Nebbioso M, Pascarella A, Cavallotti C, Pescosolido N (December 2012). "Monoamine oxidase enzymes and oxidative stress in the rat optic nerve: age-related changes". Int J Exp Pathol 93 (6): 401–5. doi:10.1111/j.1365-2613.2012.00832.x. PMC 3521895. PMID 23082958.
- ↑ Kitani K, Kanai S, Sato Y, Ohta M, Ivy GO, Carrillo MC (1993). "Chronic treatment of (-)deprenyl prolongs the life span of male Fischer 344 rats. Further evidence". Life Sci. 52 (3): 281–8. doi:10.1016/0024-3205(93)90219-S. PMID 8423709.
- ↑ Lenders JW, Eisenhofer G, Abeling NG, Berger W, Murphy DL, Konings CH, Wagemakers LM, Kopin IJ, Karoum F, van Gennip AH, Brunner HG (February 1996). "Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes". J. Clin. Invest. 97 (4): 1010–9. doi:10.1172/JCI118492. PMC 507147. PMID 8613523.
- ↑ Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ↑ Miklya I (December 2009). "[Slowing the age-induced decline of brain function with prophylactic use of (−)-deprenyl (Selegiline, Jumex). Current international view and conclusions 25 years after the Knoll's proposal]". Neuropsychopharmacol Hung (in Hungarian) 11 (4): 217–25. PMID 20150659.
- ↑ Ukraintseva SV, Arbeev KG, Michalsky AI, Yashin AI (June 2004). "Antiaging treatments have been legally prescribed for approximately thirty years". Ann. N. Y. Acad. Sci. 1019: 64–9. doi:10.1196/annals.1297.014. PMID 15246996.
- 1 2 3 Novaroli L, Daina A, Favre E, Bravo J, Carotti A, Leonetti F, Catto M, Carrupt PA, Reist M (October 2006). "Impact of species-dependent differences on screening, design, and development of MAO B inhibitors". J. Med. Chem. 49 (21): 6264–72. doi:10.1021/jm060441e. PMID 17034132.
- ↑ Carotti A, Carrieri A, Chimichi S, Boccalini M, Cosimelli B, Gnerre C, Carotti A, Carrupt PA, Testa B (December 2002). "Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. Synthesis and SAR studies". Bioorg. Med. Chem. Lett. 12 (24): 3551–5. doi:10.1016/S0960-894X(02)00798-9. PMID 12443774.
- ↑ Uebelhack R, Franke L, Schewe HJ (September 1998). "Inhibition of platelet MAO-B by kava pyrone-enriched extract from Piper methysticum Forster (kava-kava)". Pharmacopsychiatry 31 (5): 187–92. doi:10.1055/s-2007-979325. PMID 9832350.
- ↑ Dhingra, Dinesh; Kumar, Vaibhav (2008-08-01). "Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of garlic extract in mice". Indian Journal of Pharmacology 40 (4): 175–179. doi:10.4103/0253-7613.43165. ISSN 0253-7613. PMC 2792615. PMID 20040952.
- ↑ Leonetti F, Capaldi C, Pisani L, Nicolotti O, Muncipinto G, Stefanachi A, Cellamare S, Caccia C, Carotti A (October 2007). "Solid-phase synthesis and insights into structure-activity relationships of safinamide analogues as potent and selective inhibitors of type B monoamine oxidase". Journal of Medicinal Chemistry 50 (20): 4909–16. doi:10.1021/jm070725e. PMID 17824599.
- ↑ compound #2d, Frédérick R, Dumont W, Ooms F, Aschenbach L, Van der Schyf CJ, Castagnoli N, Wouters J, Krief A (June 2006). "Synthesis, structural reassignment, and biological activity of type B MAO inhibitors based on the 5H-indeno[1,2-c]pyridazin-5-one core". J. Med. Chem. 49 (12): 3743–7. doi:10.1021/jm051091j. PMID 16759116.
- ↑ Carotti A, Catto M, Leonetti F, Campagna F, Soto-Otero R, Méndez-Alvarez E, Thull U, Testa B, Altomare C (November 2007). "Synthesis and monoamine oxidase inhibitory activity of new pyridazine-, pyrimidine- and 1,2,4-triazine-containing tricyclic derivatives". Journal of Medicinal Chemistry 50 (22): 5364–71. doi:10.1021/jm070728r. PMID 17910428.
- ↑ Chimenti F, Fioravanti R, Bolasco A, Chimenti P, Secci D, Rossi F, Yáñez M, Orallo F, Ortuso F, Alcaro S (May 2009). "Chalcones: a valid scaffold for monoamine oxidases inhibitors". J. Med. Chem. 52 (9): 2818–24. doi:10.1021/jm801590u. PMID 19378991.
- ↑ compound #21, Silvestri R, La Regina G, De Martino G, Artico M, Befani O, Palumbo M, Agostinelli E, Turini P (March 2003). "Simple, potent, and selective pyrrole inhibitors of monoamine oxidase types A and B". J. Med. Chem. 46 (6): 917–20. doi:10.1021/jm0256124. PMID 12620068.
- ↑ compound # (R)-8b, Chimenti F, Secci D, Bolasco A, Chimenti P, Granese A, Carradori S, Yáñez M, Orallo F, Sanna ML, Gallinella B, Cirilli R (September 2010). "Synthesis, stereochemical separation, and biological evaluation of selective inhibitors of human MAO-B: 1-(4-arylthiazol-2-yl)-2-(3-methylcyclohexylidene)hydrazines". J. Med. Chem. 53 (17): 6516–20. doi:10.1021/jm100120s. PMID 20715818.
- ↑ compound #18, Chimenti F, Maccioni E, Secci D, Bolasco A, Chimenti P, Granese A, Befani O, Turini P, Alcaro S, Ortuso F, Cardia MC, Distinto S (February 2007). "Selective inhibitory activity against MAO and molecular modeling studies of 2-thiazolylhydrazone derivatives". J. Med. Chem. 50 (4): 707–12. doi:10.1021/jm060869d. PMID 17253676.
- ↑ compound #3g, Chimenti F, Fioravanti R, Bolasco A, Manna F, Chimenti P, Secci D, Befani O, Turini P, Ortuso F, Alcaro S (February 2007). "Monoamine oxidase isoform-dependent tautomeric influence in the recognition of 3,5-diaryl pyrazole inhibitors". J. Med. Chem. 50 (3): 425–8. doi:10.1021/jm060868l. PMID 17266193.
- ↑ compound #(S)-1, Chimenti F, Maccioni E, Secci D, Bolasco A, Chimenti P, Granese A, Befani O, Turini P, Alcaro S, Ortuso F, Cirilli R, La Torre F, Cardia MC, Distinto S (November 2005). "Synthesis, molecular modeling studies, and selective inhibitory activity against monoamine oxidase of 1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)- pyrazole derivatives". J. Med. Chem. 48 (23): 7113–22. doi:10.1021/jm040903t. PMID 16279769.
- ↑ Mishra N, Sasmal D (April 2011). "Development of selective and reversible pyrazoline based MAO-B inhibitors: virtual screening, synthesis and biological evaluation". Bioorg. Med. Chem. Lett. 21 (7): 1969–73. doi:10.1016/j.bmcl.2011.02.030. PMID 21377879.
- ↑ compound #41, Catto M, Nicolotti O, Leonetti F, Carotti A, Favia AD, Soto-Otero R, Méndez-Alvarez E, Carotti A (2006). "Structural insights into monoamine oxidase inhibitory potency and selectivity of 7-substituted coumarins from ligand- and target-based approaches". Journal of Medicinal Chemistry 49 (16): 4912–25. doi:10.1021/jm060183l. PMID 16884303.
- ↑ compound #2, Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L, Viña D (July 2011). "MAO inhibitory activity modulation: 3-Phenylcoumarins versus 3-benzoylcoumarins". Bioorg. Med. Chem. Lett. 21 (14): 4224–7. doi:10.1016/j.bmcl.2011.05.074. PMID 21684743.
- ↑ Matos MJ, Viña D, Janeiro P, Borges F, Santana L, Uriarte E (September 2010). "New halogenated 3-phenylcoumarins as potent and selective MAO-B inhibitors". Bioorg. Med. Chem. Lett. 20 (17): 5157–60. doi:10.1016/j.bmcl.2010.07.013. PMID 20659799.
- ↑ Matos MJ, Viña D, Picciau C, Orallo F, Santana L, Uriarte E (September 2009). "Synthesis and evaluation of 6-methyl-3-phenylcoumarins as potent and selective MAO-B inhibitors". Bioorg. Med. Chem. Lett. 19 (17): 5053–5. doi:10.1016/j.bmcl.2009.07.039. PMID 19628387.
- ↑ Matos MJ, Viña D, Quezada E, Picciau C, Delogu G, Orallo F, Santana L, Uriarte E (June 2009). "A new series of 3-phenylcoumarins as potent and selective MAO-B inhibitors". Bioorg. Med. Chem. Lett. 19 (12): 3268–70. doi:10.1016/j.bmcl.2009.04.085. PMID 19423346.
- ↑ compound #9, #12, Gaspar A, Reis J, Fonseca A, Milhazes N, Viña D, Uriarte E, Borges F (January 2011). "Chromone 3-phenylcarboxamides as potent and selective MAO-B inhibitors". Bioorg. Med. Chem. Lett. 21 (2): 707–9. doi:10.1016/j.bmcl.2010.11.128. PMID 21194943.
- ↑ compound #9i, Manley-King CI, Bergh JJ, Petzer JP (January 2011). "Inhibition of monoamine oxidase by selected C5- and C6-substituted isatin analogues". Bioorg. Med. Chem. 19 (1): 261–74. doi:10.1016/j.bmc.2010.11.028. PMID 21134756.
- ↑ compound #5c, Manley-King CI, Bergh JJ, Petzer JP (August 2011). "Inhibition of monoamine oxidase by C5-substituted phthalimide analogues". Bioorg. Med. Chem. 19 (16): 4829–40. doi:10.1016/j.bmc.2011.06.070. PMID 21778064.
- ↑ Strydom B, Bergh JJ, Petzer JP (August 2011). "8-Aryl- and alkyloxycaffeine analogues as inhibitors of monoamine oxidase". Eur J Med Chem 46 (8): 3474–85. doi:10.1016/j.ejmech.2011.05.014. PMID 21621312.
- ↑ Strydom B, Malan SF, Castagnoli N, Bergh JJ, Petzer JP (February 2010). "Inhibition of monoamine oxidase by 8-benzyloxycaffeine analogues". Bioorg. Med. Chem. 18 (3): 1018–28. doi:10.1016/j.bmc.2009.12.064. PMID 20093036.
- ↑ Vlok N, Malan SF, Castagnoli N, Bergh JJ, Petzer JP (May 2006). "Inhibition of monoamine oxidase B by analogues of the adenosine A2A receptor antagonist (E)-8-(3-chlorostyryl)caffeine (CSC)". Bioorg. Med. Chem. 14 (10): 3512–21. doi:10.1016/j.bmc.2006.01.011. PMID 16442801.
- ↑ Pretorius J, Malan SF, Castagnoli N, Bergh JJ, Petzer JP (September 2008). "Dual inhibition of monoamine oxidase B and antagonism of the adenosine A(2A) receptor by (E,E)-8-(4-phenylbutadien-1-yl)caffeine analogues". Bioorganic & Medicinal Chemistry 16 (18): 8676–84. doi:10.1016/j.bmc.2008.07.088. PMID 18723354.
- ↑ Tzvetkov; et al. (June 23, 2014). "Indazole- and Indole-5-carboxamides: Selective and Reversible Monoamine Oxidase B Inhibitors with Subnanomolar Potency". Journal of Medicinal Chemistry 57 (15): 6679-6703. doi:10.1021/jm500729a.
Further reading
- Edmondson DE, Binda C, Mattevi A (2004). "The FAD binding sites of human monoamine oxidases A and B". Neurotoxicology 25 (1–2): 63–72. doi:10.1016/S0161-813X(03)00114-1. PMID 14697881.
- Kumar MJ, Andersen JK (2004). "Perspectives on MAO-B in aging and neurological disease: where do we go from here?". Mol. Neurobiol. 30 (1): 77–89. doi:10.1385/MN:30:1:077. PMID 15247489.
- Ghozlan A, Munnich A (2004). "[MAOB: a modifier gene in phenylketonuria?]" [MAOB: a modifier gene in phenylketonuria?]. Med Sci (Paris) (in French) 20 (10): 929–32. doi:10.1051/medsci/20042010929. PMID 15461973.
- Zhu QS, Grimsby J, Chen K, Shih JC (1992). "Promoter organization and activity of human monoamine oxidase (MAO) A and B genes". J. Neurosci. 12 (11): 4437–46. PMID 1432104.
- Grimsby J, Chen K, Wang LJ, Lan NC, Shih JC (May 1991). "Human monoamine oxidase A and B genes exhibit identical exon-intron organization". Proc. Natl. Acad. Sci. U.S.A. 88 (9): 3637–41. doi:10.1073/pnas.88.9.3637. PMC 51507. PMID 2023912.
- Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, Seeburg PH, Shih JC (July 1988). "cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties". Proc. Natl. Acad. Sci. U.S.A. 85 (13): 4934–8. doi:10.1073/pnas.85.13.4934. PMC 280552. PMID 3387449.
- Kochersperger LM, Parker EL, Siciliano M, Darlington GJ, Denney RM (1986). "Assignment of genes for human monoamine oxidases A and B to the X chromosome". J. Neurosci. Res. 16 (4): 601–16. doi:10.1002/jnr.490160403. PMID 3540317.
- Wyatt RJ, Murphy DL, Belmaker R, Cohen S, Donnelly CH, Pollin W (March 1973). "Reduced monoamine oxidase activity in platelets: a possible genetic marker for vulnerability to schizophrenia". Science 179 (4076): 916–8. doi:10.1126/science.179.4076.916. PMID 4687789.
- Goldin LR, Gershon ES, Lake CR, Murphy DL, McGinniss M, Sparkes RS (March 1982). "Segregation and linkage studies of plasma dopamine-beta-hydroxylase (DBH), erythrocyte catechol-O-methyltransferase (COMT), and platelet monoamine oxidase (MAO): possible linkage between the ABO locus and a gene controlling DBH activity". Am. J. Hum. Genet. 34 (2): 250–62. PMC 1685293. PMID 6951409.
- Denney RM, Fritz RR, Patel NT, Abell CW (1982). "Human liver MAO-A and MAO-B separated by immunoaffinity chromatography with MAO-B-specific monoclonal antibody". Science 215 (4538): 1400–3. doi:10.1126/science.7063850. PMID 7063850.
- Raddatz R, Parini A, Lanier SM (1996). "Imidazoline/guanidinium binding domains on monoamine oxidases. Relationship to subtypes of imidazoline-binding proteins and tissue-specific interaction of imidazoline ligands with monoamine oxidase B". J. Biol. Chem. 270 (46): 27961–8. doi:10.1074/jbc.270.46.27961. PMID 7499273.
- Woo JC, Silverman RB (1994). "Observation of two different chromophores in the resting state of monoamine oxidase B by fluorescence spectroscopy". Biochem. Biophys. Res. Commun. 202 (3): 1574–8. doi:10.1006/bbrc.1994.2111. PMID 8060341.
- Wu HF, Chen K, Shih JC (1993). "Site-directed mutagenesis of monoamine oxidase A and B: role of cysteines". Mol. Pharmacol. 43 (6): 888–93. PMID 8316221.
- Chen K, Wu HF, Shih JC (1993). "The deduced amino acid sequences of human platelet and frontal cortex monoamine oxidase B are identical". J. Neurochem. 61 (1): 187–90. doi:10.1111/j.1471-4159.1993.tb03554.x. PMID 8515265.
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R (February 1996). "Inhibition of monoamine oxidase B in the brains of smokers". Nature 379 (6567): 733–6. doi:10.1038/379733a0. PMID 8602220.
- Lenders JW, Eisenhofer G, Abeling NG, Berger W, Murphy DL, Konings CH, Wagemakers LM, Kopin IJ, Karoum F, van Gennip AH, Brunner HG (February 1996). "Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes". J. Clin. Invest. 97 (4): 1010–9. doi:10.1172/JCI118492. PMC 507147. PMID 8613523.
- Cesura AM, Gottowik J, Lahm HW, Lang G, Imhof R, Malherbe P, Röthlisberger U, Da Prada M (March 1996). "Investigation on the structure of the active site of monoamine oxidase-B by affinity labeling with the selective inhibitor lazabemide and by site-directed mutagenesis". Eur. J. Biochem. 236 (3): 996–1002. doi:10.1111/j.1432-1033.1996.00996.x. PMID 8665924.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Saura J, Bleuel Z, Ulrich J, Mendelowitsch A, Chen K, Shih JC, Malherbe P, Da Prada M, Richards JG (February 1996). "Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry". Neuroscience 70 (3): 755–74. doi:10.1016/S0306-4522(96)83013-2. PMID 9045087.
- Checkoway H, Franklin GM, Costa-Mallen P, Smith-Weller T, Dilley J, Swanson PD, Costa LG (May 1998). "A genetic polymorphism of MAO-B modifies the association of cigarette smoking and Parkinson's disease". Neurology 50 (5): 1458–61. doi:10.1212/WNL.50.5.1458. PMID 9596006.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||