Lithium–air battery
| Specific energy | 11,140 (theoretical) W·h/kg |
|---|---|
| Energy density | ??? |
| Specific power | ??? |
| Nominal cell voltage | 2.91 V |
The lithium-air battery, Li-air for short, is a metal-air battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.[1]
Originally proposed in the 1970s as a possible power source for battery electric vehicles, Li-air batteries recaptured scientific interest in the late 2000s due to advances in materials technology and an increasing demand for renewable energy sources.
Pairing lithium and oxygen (from air) can theoretically lead to electrochemical cells with the highest specific energy possible. Indeed, the theoretical specific energy of a non-aqueous Li-air battery (in the charged state with Li2O2 product and excluding the oxygen mass) is ~12 kWh/kg 1-2. This is comparable with the theoretical specific energy of gasoline (~13 kWh/kg 3). In practice, the Li-air batteries with a specific energy of ~1.7 kWh/kg at the cell level have been demonstrated 4-5, which is about 5 times greater than that of commercial lithium-ion batteries, and which is sufficient to run a Fully Electric Vehicle (FEV) for 500 km (300 miles) on a single charge 1, 3. (A 700 Wh/kg battery with an electric engine would be comparable with the internal combustion engine system in terms of the driving range per kg 8 while lithium ion batteries have only 105 Wh/kg at the pack level 2, 5, i.e. they are limited to < 150 km driving range).5 However, the areal power and cycle life of lithium –oxygen/air batteries need significant improvements before they can find any competitive market niche.
Significant advances in multiple fields are necessary to develop a commercial implementation.[2] Four approaches are active: aprotic,[3][4][5] aqueous,[6] solid state[7] and mixed aqueous/aprotic.[8]
Metal-air batteries, specifically zinc-air, have received attention due to potentially high energy densities. The theoretical specific energy densities for metal-air batteries are higher than for ion-based approaches. Lithium-air batteries can theoretically achieve 3840 mA·h/g.[9]
A major driver is the automotive sector. The energy density of gasoline is approximately 13 kW·h/kg, which corresponds to 1.7 kW·h/kg of energy provided to the wheels after losses. Theoretically lithium-air can achieve 12 kW·h/kg (43.2 MJ/kg) excluding the oxygen mass and deliver the same 1.7 kW·h/kg to the wheels, after losses from over-potentials, other cell components and battery pack auxiliaries, given the much higher efficiency of electric motors.[10]
Lithium-air batteries have the potential of 5–15 times the specific energy of current lithium-ion batteries.[11]
History
Although, the idea of lithium-air battery was around long before 1996 (Abraham and Jiang 1996, Lu and Amine 2013, Balaish, Kraytsberg et al. 2014, Lu, Li et al. 2014), the risks-to-benefits ratio for such technology was not perceived at that time as making it worth pursuing. Indeed, both the negative (lithium metal) and the positive (air or oxygen electrodes) are the reasons why, respectively, rechargeable lithium-metal batteries failed to get on the market in the 1970 (lithium-ion battery in your cell phone uses a LiC6 -graphite compound on the negative electrode, not a lithium metal), and fuel cells failed during Hydrogen Economy epoch (2001-2009 in the USA). Nevertheless, due to the a perceived lack of other alternatives to high specific energy rechargeable batteries, and due to some initially promising results from academic labs (Abraham and Jiang 1996, Lu and Amine 2013), both the number of patents and of free-domain publications related to lithium-oxygen (including Li-air) batteries has been growing exponentially since ca. 2006 (Ogasawara, Débart et al. 2006, Lu and Amine 2013) (see Fig. 1). (The drop in the apparent patent number in the last 2 years is due to a delay between the priority and publication dates. For example, there is a 30 month delay between a Provisional Patent application being filed and a Patent Cooperation Treaty application being published.) However, the technical difficulties facing such batteries, especially considering that they have to be electrically rechargeable for most applications, are daunting (Lee, Roev et al. 2015).
Operation

Although details vary by battery design, in general, lithium ions move between the anode and the cathode sides across the electrolyte. Under discharge, electrons follow the external circuit to do electric work and the lithium ions migrate across the electrolyte. During charge (when an external potential becomes greater than the standard potential for the discharge reaction), the lithium metal plates onto the anode, freeing O
2 at the cathode.[12]
Both non-aqueous(McCloskey, Burke et al. 2015) (with Li2O2 or LiO2 as the discharge products) and aqueous (LiOH as the discharge product) Li-O2 batteries have been considered.(Balaish, Kraytsberg et al. 2014, Imanishi and Yamamoto 2014) The aqueous battery requires a protective layer on the negative electrode to keep the Li metal from reacting with water.

Anode
Lithium metal is the typical anode choice. At the anode, electrochemical potential forces the lithium metal to give off electrons via oxidation (without involving the cathodic oxygen). The half reaction is:[13]
- Li ↔ Li+ + e−
Lithium has high specific capacity (3840 mAh/g) compared with other metal-air battery materials (820 mAh/g for Zinc, 2965 mAh/g for aluminium).[14] Several issues affect such cells.
Upon charging/discharging in aprotic cells, layers of lithium salts precipitate onto the anode, eventually covering it and creating a barrier between the lithium and electrolyte. This barrier initially prevents corrosion, but eventually inhibits the reaction kinetics between the anode and the electrolyte.[15] This chemical change of the solid-electrolyte interface (SEI) results in varying chemical composition across the surface, causing the current to vary from point to point. The uneven current distribution furthers branching dendrite growth and typically leads to a short circuit between the anode and cathode.[16]
In aqueous cells problems at the SEI stem from the high reactivity of lithium metal with water.[17]
Several approaches have been taken to overcome problems at the SEI:
- Formation of a Li-ion protective layer using di- and triblock copolymer electrolytes.[18] According to Seeo, Inc.,[18] such electrolytes (e.g. polystyrene with the high Li-ion conductivity of a soft polymer segment, such as a poly(ethylene oxide PEO/ Li-salt mixture) ) combine the mechanical stability of a hard polymer segment with the high ionic conductivity of the soft polymer/lithium salt mixture. The hardness inhibits dendrite shorts via mechanical blocking.
- Li-ion conducting glass or glass-ceramic materials[7][19][20] are (generally) readily reduced by lithium metal, and therefore a thin film of a stable lithium conducting material, such as Li
3P or Li
3N, can be inserted between the ceramic and metal. This ceramic-based SEI inhibits the formation of dendrites and protects the lithium metal from atmospheric contamination.
Cathode and electrolyte
At the cathode during charge, oxygen donates electrons to the lithium via reduction. Mesoporous carbon has been used as a cathode substrate with metal catalysts[21] that enhance reduction kinetics and increase the cathode's specific capacity.[11] Manganese, cobalt, ruthenium, platinum, silver, or a mixture of cobalt and manganese are under consideration as metal catalysts. Under some circumstances manganese-catalyzed cathodes performed best, with a specific capacity of 3137 mA·H/g carbon and cobalt-catalyzed cathodes performed second best, with a specific capacity of 2414 mA·H/g carbon.[22] Based on the first pore-scale modeling of lithium-air batteries, the microstructure of the cathode significantly affects battery capacity in both non-pore-blocking and pore-blocking regimes.[23]
Li-air cell performance is limited by the efficiency of reaction at the cathode because most of the voltage drop occurs there.[14] Multiple battery chemistries have been assessed, distinguished by electrolyte. This discussion focuses on aprotic and aqueous electrolytes as the solid-state electrochemistry is not well understood.
In a cell with an aprotic electrolyte lithium oxides are produced through reduction at the cathode:
- Li+ + e− +O
2 + * → LiO
2* - Li+ + e− +LiO
2* →Li
2O
2*
where "*" denotes a surface site on Li
2O
2 where growth proceeds, which is essentially a neutral Li vacancy in the Li
2O
2 surface.
Lithium oxides are insoluble in aprotic electrolytes, which leads to cathode clogging.[24]
In a cell with an aqueous electrolyte the reduction at the cathode can also produce lithium hydroxide:
Acidic electrolyte
- 2Li + 1⁄2O
2 + 2H+ → 2Li++ H
2O
A conjugate base is involved in the reaction. The theoretical maximal Li-air cell specific energy and Li-air cell energy density is 1400 W·h/kg and 1680 W·h/l, respectively.[10]
Alkaline aqueous electrolyte
- 2Li + 1⁄2O
2 + H
2O → 2LiOH
Water molecules are involved in the redox reactions at the air cathode. The theoretical maximal Li-air cell specific energy and Li-air cell energy density is 1300 W·h/kg and 1520 W·h/l, respectively.[10]
The development of new cathode materials must account for the accommodation of substantial amounts of LiO
2,Li
2O
2 and/or LiOH without causing the cathode pores to block and employ suitable catalysts to make the electrochemical reactions energetically practical.
- Dual pore system materials offer the most promising energy capacity.[25]
- The first pore system serves as an oxidation product store.
- The second pore system serves as oxygen transport.
Design
Cathode
A MnO
2 nanowire array cathode augmented by a genetically modified M13 virus offers two to three times the energy density of 2015 lithium-ion batteries. The virus increased the size of the nanowire array, which is about 80 nm across. The resulting wires had a spiked surface. Spikes create more surface area to host reaction sites. The viral process creates a cross-linked 3D structure, rather than isolated wires, stabilizing the electrode. The viral process is water-based and takes place at room temperature.[26][27]
Electrolyte
Efforts in Li-air batteries have focused on four different chemical designs. All the designs have distinct advantages and significant technical challenges.
Aprotic

The non-aqueous Li-air batteries were demonstrated first (Abraham and Jiang 1996). They usually use the same mixed ethylene carbonate+ propylene carbonate solvents with LiPF4 or Li bis-sulfonimide salts like the conventional lithium-ion batteries, however, with a gelled rather than liquid electrolyte. (Imanishi, Matsui et al. 2014) The voltage difference upon constant current charge and discharge is usually between 1.3 and 1.8 V (with a OCP of ca. 4.2 V) even at such ridiculously low currents as 0.01-0.5 mA/cm2 and 50-500 mA/g of C on the positive electrode (see Fig. 2 as an example).(Balaish, Kraytsberg et al. 2014, McCloskey, Burke et al. 2015, Liu, Xu et al. 2016) However, the carbonate solvents do evaporate and get oxidized due to a high overvoltage upon charge,(Lu and Amine 2013) and other solvents, such as end-capped glymes, DMSO, dimethylacetamide, and ionic liquids, have been considered.(Balaish, Kraytsberg et al. 2014, Imanishi, Matsui et al. 2014) Carbon cathode also gets oxidized above +3.5 V v Li during charge forming Li2CO3, which leads to an irreversible capacity loss.(Imanishi, Matsui et al. 2014)
Most effort involved aprotic materials, which consist of a lithium metal anode, a liquid organic electrolyte and a porous carbon cathode.[3] Electrolytes can be made of any organic capable of solvating lithium salts such as LiPF
6, LiAsF
6, LiN(SO
2CF
3)
2, and LiSO
3CF
3), but typically consisted of carbonates, ethers and esters.[3][12] The carbon cathode is usually made of a high-surface-area carbon material with a nanostructured metal oxide catalyst (commonly MnO
2 or Mn
3O
4). A major advantage is the spontaneous formation of a barrier between anode and electrolyte (analogous to the barrier formed between electrolyte and carbon-lithium anodes in conventional Li-ion batteries) that protects the lithium metal from further reaction with the electrolyte. Although rechargeable,[10] the Li
2O
2 produced at the cathode is generally insoluble in the organic electrolyte, leading to buildup along the cathode/electrolyte interface. This makes cathodes in aprotic batteries prone to clogging and volume expansion that progressively reduces conductivity and degrades battery performance.[6][17][28] Another issue is that organic electrolytes are flammable and can ignite if the cell is damaged.[7]
In 2012, researchers announced that a dimethyl sulfoxide electrolyte and gold nanoparticles cathode achieved 100 charge cycles with 5% capacity loss.[29]
Although most studies agree that Li2O2 is the final discharge product of non-aqueous Li-O2 batteries, there is a considerable body of evidence that its formation does not proceed as a direct 2-electron electroreduction to peroxide O22- (which is the common pathway for O2 reduction in water on carbon) but rather via a 1 – electron reduction to superoxide O2- , followed but its disproportionation:
2LiO2 = Li2O2+O2 (1).
Superoxide (O2-) has been traditionally considered as a dangerous intermediate in aprotic oxygen batteries due to its high nucleophilicity, basicity and redox potential.(Balaish, Kraytsberg et al. 2014, McCloskey, Burke et al. 2015) However, recent reports from Argonne (Zhai, Lau et al. 2015, Lu, Lee et al. 2016) suggest that superoxide (LiO2 ) is not just an intermediate during the discharge to peroxide (Li2O2 ) and but that that LiO2 it can actually be used as the final discharge product, potentially with an improved cycle life albeit with a lower specific energy (a little heavier battery weight). Indeed, it was shown that under certain conditions the superoxide can be stable on the scale of 20-70 h at room temperature.(Zhai, Lau et al. 2015) Although an irreversible capacity loss upon disproportionation of LiO2 in the charged battery was not addressed in that work.
Pt/C seems to be the best electrocatalysts for O2 evolution and Au/C for O2 reduction when Li2O2 is the product.(Lu, Xu et al. 2010) Nevertheless, “the performance of rechargeable lithium-air batteries with non-aqueous electrolytes is limited by the reactions on the oxygen electrode, especially by O2 evolution… Conventional porous carbon air electrodes are unable to provide mAh/g and mAh/cm2 capacities and discharge rates at the magnitudes required for really high energy density batteries for EV applications.” (Lu, Xu et al. 2010) The capacity (in mAh/cm2) and the cycle life of the non-aqueous Li-O2 batteries is limited by the deposition of insoluble and poorly electronically conducting LiOx phases upon discharge.(Balaish, Kraytsberg et al. 2014) (It is worth noting that Li3O4 is predicted to have a better Li+ conductivity that the LiO2 and Li2O2 phases).(Shi, Xu et al. 2015) This makes the practical specific energy of Li-O2 batteries significantly smaller than the reagent-level calculation predicts. It seems that these parameters reached their limitations by now, and further improvement can be expected only from alternative approaches.

Aqueous
An aqueous Li-air battery consists of a lithium metal anode, an aqueous electrolyte and a porous carbon cathode. The aqueous electrolyte combines lithium salts dissolved in water. It avoids the issue of cathode clogging because the reaction products are water-soluble.[6] The aqueous design has a higher practical discharge potential than its aprotic counterpart. However, lithium metal reacts violently with water and thus the aqueous design requires a solid electrolyte interface between the lithium and electrolyte. Commonly, a lithium-conducting ceramic or glass is used, but conductivities are generally low (on the order of 10−3 S/cm at ambient temperatures).[17]

Mixed aqueous/aprotic
The aqueous/aprotic or mixed Li-air battery design attempts to unite advantages of the aprotic and aqueous battery designs. The common feature of hybrid designs is a two-part (one part aqueous and one part aprotic) electrolyte connected by a lithium-conducting membrane. The anode abuts the aprotic side while the cathode is in contact with the aqueous side. A lithium-conducting ceramic is typically employed as the membrane joining the two electrolytes.[6][10]
The use of a solid electrolyte (see Fig. 3) is one such alternative approaches that allows for a combination of a lithium metal anode with an aqueous cathode.(Visco 2004) Ceramic solid electrolytes (CSEs) of the NASICON type (e.g. Li1-xAxM2-x(PO4)3 with A∈ [Al, Sc, Y] and M ∈ [Ti, Ge]) is one family of Li+ conducting materials that has been studied. Albeit compatible with water at alkaline pH and having a large electrochemical window (see Figs. 3,4), their low Li+ ion conductivity near room temperature (< 0.005 S/cm, >85 Ω cm2) (Imanishi, Matsui et al. 2014) makes them unsuitable for automotive and stationary energy storage applications which demand a low cost of power (i.e. operating current densities over 100 mA/cm2). Furthermore both Ti and Ge are reduced by metallic Li, and an intermediate layer between the ceramic electrode and the negative electrode is required. On the other hand, solid polymer electrolytes (SPEs) can provide a higher conductivity but at the expense of a faster crossover of water and of other small molecules which are reactive toward metallic Li. Among the more exotic membranes considered for Li-O2 batteries is single-crystal silicon (Lu and Amine 2013).
In 2015 researchers announced a design that a used highly porous form of graphene for the anode, an electrolyte of lithium bis(trifluoromethyl) sulfonylimide/dimethoxyethane with added water and lithium iodide for use as a "mediator". The electrolyte produces lithium hydroxide (LiOH) at the cathode instead of lithium peroxide (Li2O2). The result offered energy efficiency of 93 percent (voltage gap of .2) and cycled more than 2,000 times with little impact on output.[30][31] However, the design required pure oxygen to function, rather than ambient air.[32]
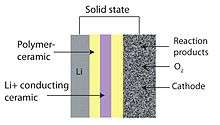
Solid state
The solid-state battery design is attractive from a safety standpoint, eliminating the possibility of ignition from rupture.[7] Current solid-state Li-air batteries use a lithium anode, a ceramic, glass, or glass-ceramic electrolyte, and a porous carbon cathode. The anode and cathode are typically separated from the electrolyte by polymer-ceramic composites that enhance charge transfer at the anode and electrochemically couple the cathode to the electrolyte. The polymer-ceramic composites reduce overall impedance. The main drawback of the solid-state battery design is the low conductivity of most glass-ceramic electrolytes. The ionic conductivity of current lithium fast ion conductors is still lower than liquid electrolyte alternatives.[9]
Challenges
As of 2013 many challenges confronted designers of Li-air batteries.
Cathode
Most of the current limitations in Li-air battery development are at the cathode, which is also the source of its potential advantages. Incomplete discharge due to blockage of the porous carbon cathode with discharge product such as lithium peroxide (in aprotic designs) is the most serious.
The effect of pore size and pore size distribution remains poorly understood.[14]
Catalysts have shown promise in creating preferential nucleation of Li
2O
2 over Li
2O, which is irreversible with respect to lithium.[33]
Atmospheric oxygen must be present at the cathode, but contaminants such as water vapor can damage it.[4]
Anode
The main challenge in anode development is preventing the anode from reacting with the electrolyte. Alternatives include new electrolyte materials or redesigning the interface between electrolyte and anode.
Dendritic lithium deposits can decrease energy capacity or trigger a short circuit.
Electrochemical
In current cell designs, the charge overpotential is much higher than the discharge overpotential. Significant charge overpotential indicates the presence of secondary reactions.[34] As a result, electrical efficiency is only around 65%.[14]
Catalysts such as MnO
2, Co, Pt and Au can potentially reduce the overpotentials, but the effect is poorly understood.[33] Several catalysts improve cathode performance, notably MnO
2. The mechanism of improvement is unknown, but may alter the structure of the oxide deposits.[35][36]
Significant drops in cell capacity with increasing discharge rates are another issue. The decrease in cell capacity is attributed to kinetic charge transfer limitations.[14] Since the anodic reaction occurs very quickly, the charge transfer limitations are thought to occur at the cathode.
Stability
Long term battery operation requires chemical stability of all cell components. Current cell designs show poor resistance to oxidation by reaction products and intermediates. Many aqueous electrolytes are volatile and can evaporate over time.[14]
Applications
Vehicles
Li-air cells feature high specific and volumetric energy density, comparable to petrol. Electric motors provide high efficiency (95% compared to 35% for an internal combustion engine. Thus, Li-air cells could offer range equivalent to today's vehicles with a battery pack 1/3 the size of standard fuel tanks. The reduced vehicle weight creates a virtuous circle in that the motor size can be reduced, further reducing weight and battery requirements.
Grid backup
In 2014 researchers announced a hybrid solar cell/battery. Up to 20% of the energy produced by conventional solar cells is lost as it travels to and charges a battery. The hybrid stores nearly 100% of the energy produced. The first version of the hybrid used a potassium-air battery. It offered higher energy density than conventional Li-ion batteries, was less expensize and avoided toxic byproducts. The latest device essentially substituted lithium for potassium.[37]
The solar cell used a mesh made from microscopic rods of titanium dioxide to allow the required oxygen to pass through. Captured sunlight produced electrons that decompose lithium peroxide into lithium ions, thereby charging the battery. During discharge, oxygen from air replenished the lithium peroxide.[37]
Future Prospects
Most researches working in the field of Li-O2 batteries do not consider this technology as a good investment opportunity.
“The allure of an ultra-high –energy density battery has prompted substantial research into the Li-O2 electrochemical couple as a possible battery chemistry and a significant progress has been made over the past decade … However, expectations for a practical Li-air battery should be kept modest given the severity of the challenges facing the battery chemistry.” (UC Berkley, USA 2015).(McCloskey, Burke et al. 2015)
“During the first decade of this century, a fair amount of research has been conducted on Li-air battery system. Yet, Li-air batteries could not make an industrial breakthrough, and are still in the laboratory phase since their birth. … low discharge rate, low… number of cycles, oxidation of lithium anode, discharge products at the cathode, and side reactions inside the battery are the key limiting factors in the slow progress of Li-air batteries on an industrial scale.” (Material Science and Engineering School, Beihang University, Beijing, China) (Akhtar and Akhtar 2015)
“For aqueous lithium–air batteries, the air electrode capacity is not dependent on the specific area of the carbon, but on the amount of aqueous electrolyte, assuming the reaction product of LiOH×H2O is deposited in the electrolyte. The expected energy density for non-aqueous and aqueous lithium–air batteries could be acceptable for EV applications; however, such batteries must be capable of being discharged and charged at the rate required of EV applications, which may be the most challenging target yet.” “No technological basis exists to support the highly optimistic energy densities projected for lithium-air batteries. Moreover, capabilities for high power density and extended deep cycling have not been shown.” (Imanishi, Matsui et al. 2014) (Mie University, Japan) Another recent review on Li-O2 batteries, authored by materials scientists from Technion- Israel Institute of Technology (Balaish, Kraytsberg et al. 2014), concludes with : “The possibility of buying off the shelf Li–air batteries within 10–20 years does not seem realistic at the moment.”
See also
References
- ↑ Badwal, Sukhvinder P. S.; Giddey, Sarbjit S.; Munnings, Christopher; Bhatt, Anand I.; Hollenkamp, Anthony F. (24 September 2014). "Emerging electrochemical energy conversion and storage technologies". Frontiers in Chemistry 2. doi:10.3389/fchem.2014.00079.
- ↑ Christensen, J.; Albertus, P.; Sanchez-Carrera, R. S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. (2012). "A Critical Review of Li∕Air Batteries". Journal of the Electrochemical Society 159 (2): R1. doi:10.1149/2.086202jes.
- 1 2 3 Younesi, Reza; Veith, Gabriel M.; Johansson, Patrik; Edström, Kristina; Vegge, Tejs (2015). "Lithium salts for advanced lithium batteries: Li–metal, Li–O 2 , and Li–S". Energy Environ. Sci. 8 (7): 1905–1922. doi:10.1039/c5ee01215e.
- 1 2 Ogasawara, T.; Débart, A. L.; Holzapfel, M.; Novák, P.; Bruce, P. G. (2006). "Rechargeable Li2O2Electrode for Lithium Batteries". Journal of the American Chemical Society 128 (4): 1390–1393. doi:10.1021/ja056811q. PMID 16433559.
- ↑ Debart A, Bao,J et al (2008). "α-MnO
2 Nanowires: A Catalyst for theO
2 Electrode in Rechargeable Lithium Batteries",Angew.Chem.,47(24):p.4521-4524. - 1 2 3 4 He, P.; Wang, Y.; Zhou, H. (2010). "A Li-air fuel cell with recycle aqueous electrolyte for improved stability". Electrochemistry Communications 12 (12): 1686. doi:10.1016/j.elecom.2010.09.025.
- 1 2 3 4 Kumar, B.; Kumar, J.; Leese, R.; Fellner, J. P.; Rodrigues, S. J.; Abraham, K. M. (2010). "A Solid-State, Rechargeable, Long Cycle Life Lithium–Air Battery". Journal of the Electrochemical Society 157: A50. doi:10.1149/1.3256129.
- ↑ Wang, Yonggang (2010). "A lithium-air battery with a potential to continuously reduce O2 from air for delivering energy". Journal of Power Sources 195 (1): 358–361. doi:10.1016/j.jpowsour.2009.06.109.
- 1 2 Kumar, B.; Kumar, J. (2010). "Cathodes for Solid-State Lithium–Oxygen Cells: Roles of Nasicon Glass-Ceramics". Journal of the Electrochemical Society 157 (5): A611. doi:10.1149/1.3356988.
- 1 2 3 4 5 Girishkumar, G.; McCloskey, B.; Luntz, A. C.; Swanson, S.; Wilcke, W. (2010). "Lithium−Air Battery: Promise and Challenges". The Journal of Physical Chemistry Letters 1 (14): 2193. doi:10.1021/jz1005384.
- 1 2 Ed. Jurgen O. Besenhard, Handbook of Battery Materials,New Your, Wiley-VCH, 1999, ISBN 3-527-29469-4.
- 1 2 Xu, K. (2004). "Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries". Chemical Reviews 104 (10): 4303–417. doi:10.1021/cr030203g. PMID 15669157.
- ↑ Winter, M.; Brodd, R. J. (2004). "What Are Batteries, Fuel Cells, and Supercapacitors?". Chemical Reviews 104 (10): 4245–4269. doi:10.1021/cr020730k. PMID 15669155.
- 1 2 3 4 5 6 Kraytsberg, A.; Ein-Eli, Y. (2011). "Review on Li–air batteries—Opportunities, limitations and perspective". Journal of Power Sources 196 (3): 886. doi:10.1016/j.jpowsour.2010.09.031.
- ↑ Aurbach, D. (2000). "Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries". Journal of Power Sources 89 (2): 206–218. doi:10.1016/S0378-7753(00)00431-6.
- ↑ Whittingham, M. S. (1976). "Electrical Energy Storage and Intercalation Chemistry". Science 192 (4244): 1126–1127. doi:10.1126/science.192.4244.1126. PMID 17748676.
- 1 2 3 Kowalczk, I.; Read, J.; Salomon, M. (2007). "Li-air batteries: A classic example of limitations owing to solubilities". Pure and Applied Chemistry 79 (5): 851. doi:10.1351/pac200779050851.
- 1 2 Singh, M.; Gur, I.; Balsara, N. P.(2009). "Solid Electrolyte Material Manufacturable by Polymer Processing Methods", U.S. Patent Application #12271829.
- ↑ Bates, J. (2000). "Thin-film lithium and lithium-ion batteries". Solid State Ionics 135: 33–37. doi:10.1016/S0167-2738(00)00327-1.
- ↑ Visco, S.; Nimonm, Y. (2010). "Active Metal/Aqueous Electrochemical Cells and Systems", U.S.Patent #7645543.
- ↑ New Energy and Fuel. 2011. Accessed 20 Nov. 2011
- ↑ Abraham, K. M. (1996). "A Polymer Electrolyte-Based Rechargeable Lithium/Oxygen Battery". Journal of the Electrochemical Society 143: 1–0. doi:10.1149/1.1836378.
- ↑ Charles P. Andersen, Han Hu, Gang Qiu, Vibha Kalra, and Ying Sun, "Pore-Scale Transport Resolved Model Incorporating Cathode Microstructure and Peroxide Growth in Lithium-Air Batteries", J. Electrochem. Soc., 162, (2015) A1135-A1145
- ↑ Read, J. (2002). "Characterization of the Lithium/Oxygen Organic Electrolyte Battery". Journal of the Electrochemical Society 149 (9): A1190–A1196. doi:10.1149/1.1498256.
- ↑ Williford, R. E.; Zhang, J. G. (2009). "Air electrode design for sustained high power operation of Li/air batteries". Journal of Power Sources 194 (2): 1164. doi:10.1016/j.jpowsour.2009.06.005.
- ↑ "Lithium-air batteries go viral for greater durability and performance". gizmag.com.
- ↑ Oh, D.; Qi, J.; Lu, Y. C.; Zhang, Y.; Shao-Horn, Y.; Belcher, A. M. (2013). "Biologically enhanced cathode design for improved capacity and cycle life for lithium-oxygen batteries". Nature Communications 4. doi:10.1038/ncomms3756.
- ↑ Li, Xianglin; Faghri, Amir (2012). "Optimization of the Cathode Structure of Lithium-Air Batteries Based on a Two-Dimensional, Transient, Non-Isothermal Model". Journal of Electrochemical Society 159 (10): A1747–A1754. doi:10.1149/2.043210jes.
- ↑ Service, Robert F. "An Electric Car That Actually Goes Far? – ScienceNOW". News.sciencemag.org. Retrieved 2012-07-20.
- ↑ Delacey, Lynda (November 19, 2015). "More hurdles jumped on path to a practical lithium-air battery". www.gizmag.com. Retrieved 2015-12-03.
- ↑ Liu, Tao; Leskes, Michal; Yu, Wanjing; Moore, Amy J.; Zhou, Lina; Bayley, Paul M.; Kim, Gunwoo; Grey, Clare P. (2015-10-30). "Cycling Li-O2 batteries via LiOH formation and decomposition". Science 350 (6260): 530–533. doi:10.1126/science.aac7730. ISSN 0036-8075. PMID 26516278.
- ↑ "New design points a path to the 'ultimate' battery". phys.org. October 29, 2015. Retrieved 2015-12-03.
- 1 2 Lu, Yi-Chun (2010). "The Influence of Catalysts on Discharge and Charge Voltages of Rechargeable Li–Oxygen Batteries". Electrochemical and Solid-State Letters 13 (6): A69. doi:10.1149/1.3363047.
- ↑ Zhang, T.; Imanishi, N.; Shimonishi, Y.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. (2010). "A novel high energy density rechargeable lithium/air battery". Chemical Communications 46 (10): 1661–1663. doi:10.1039/b920012f. PMID 20177608.
- ↑ Darren Quick (5 April 2010). "Lithium-air batteries offer three times the energy density". Retrieved 5 Oct 2011.
- ↑ Shimonishi, Y.; Zhang, T.; Imanishi, N.; Im, D.; Lee, D. J.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. (2011). "A study on lithium/air secondary batteries—Stability of the NASICON-type lithium ion conducting solid electrolyte in alkaline aqueous solutions". Journal of Power Sources 196 (11): 5128. doi:10.1016/j.jpowsour.2011.02.023.
- 1 2 DIMBERUON, PENIEL M. (October 28, 2014). "New Hybrid Solar Cell Battery Takes Aim at Solar Power’s Energy Storage Problem". Singularity Hub. Retrieved February 2015.
- K. M. Abraham and Z. Jiang, US Patent 5,510,209 (1996)
- Abraham, K. M.; H.S. Choe; D.M. Pasquariello (1998). "Polyacrylonitrile electrolyte-based Li ion batteries". Electrochimica Acta 43 (16–17): 2399–2412. doi:10.1016/S0013-4686(97)10168-2.
- Abraham, K. M.; Z. Jiang; B. Carroll (1997). "Highly Conductive PEO-like Polymer Electrolytes". Chemistry of Materials 9 (9): 1978–1988. doi:10.1021/cm970075a.
- A. Dobley, C. Morein, R. Roark, and Kuzhikalail M. Abraham, 42nd Power Sources Conference, Philadelphia, PA, June 2006
- K.M. Abraham Ph.D. at E-KEM Sciences
External links
- Argonne opens chapter in battery research – lithium air
- Argonne advanced battery research driving to displace gasoline
- The IBM Battery 500 Project
- PolyPlus battery company
- Lithion, Inc. Lithium-air battery design
| ||||||||||||||||||||
