Mesalazine
 | |
| Systematic (IUPAC) name | |
|---|---|
|
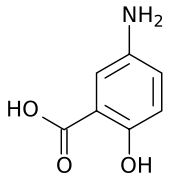
5-amino-2-hydroxybenzoic acid | |
| Clinical data | |
| Trade names | Pentasa, Delzicol, Canasa, Rowasa, Lialda, Apriso, Salofalk |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a688021 |
| Licence data | US Daily Med:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | oral, rectal |
| Pharmacokinetic data | |
| Bioavailability |
orally: 20-30% absorbed rectally: 10-35% |
| Metabolism | Rapidly & extensively metabolised intestinal mucosal wall and the liver |
| Biological half-life |
5 hours after initial dose. At steady state 7 hours |
| Identifiers | |
| CAS Number |
89-57-6 |
| ATC code | A07EC02 |
| PubChem | CID 4075 |
| IUPHAR/BPS | 2700 |
| DrugBank |
DB00244 |
| ChemSpider |
3933 |
| UNII |
4Q81I59GXC |
| KEGG |
D00377 |
| ChEBI |
CHEBI:6775 |
| ChEMBL |
CHEMBL704 |
| Chemical data | |
| Formula | C7H7NO3 |
| Molar mass | 153.135 g/mol |
| |
| |
| (verify) | |
Mesalazine (INN, BAN), also known as mesalamine (USAN) or 5-aminosalicylic acid (5-ASA), is an anti-inflammatory drug used to treat inflammatory bowel disease, such as ulcerative colitis.[1] [2] Mesalazine is a bowel-specific aminosalicylate drug that acts locally in the gut and has its predominant actions there, thereby having few systemic side effects.[3]
As a derivative of salicylic acid, mesalazine is also thought to be an antioxidant that traps free radicals, which are potentially damaging byproducts of metabolism.[3]
Mesalazine is the active moiety of sulfasalazine, which is metabolized to sulfapyridine and mesalazine.[4]
Mesalazine is the active component of the prodrug balsalazide along with the inert carrier molecule 4-aminobenzoyl-beta-alanine.[5]
Side effects
Commonly:
- Diarrhea
- Nausea
- Cramping
- Flatulence[6]
Uncommonly:
- Headache
- Exacerbation of the colitis
- Hypersensitivity reactions (including rash, urticaria aka hives, interstitial nephritis and lupus erythematosus-like syndrome)
- Hair loss
Rarely:
- Acute pancreatitis
- Hepatitis
- Nephrotic syndrome
- Blood disorders (including agranulocytosis, aplastic anaemia, leukopenia, neutropenia, thrombocytopenia)
- Fever [7]
Mesalazine avoids the sulfonamide side effects of sulfasalazine (which contains additional sulfapyridine), but carries additional rare risks of:
- Allergic lung reactions
- Allergic myocarditis
- Methaemoglobinaemia
Monitoring
As a result of the small risks of kidney, liver and blood disorders, blood tests should be taken before and after starting treatment. Patients are advised to report any unexplained bleeding, bruising, purpura, sore throat, fever or malaise that occurs during treatment so that a full blood count can be urgently taken.
Formulations

Mesalazine is formulated for oral ingestion as tablets or granules, and for rectal administration as a rectal suppository, suspension or enemas.[8] It is marketed under a variety of brand names:
- UK: Asacol, Ipocal, Pentasa, Salofalk, Mezavant XL, Octasa
- Ireland: Asacolon, Pentasa, Salofalk, Mezavant XL
- France: Asacol, Pentasa, Mezavant
- US: Asacol HD, Canasa, Rowasa, Pentasa, Delzicol, Lialda, Apriso, Salofalk
- Spain / España: Pentasa, Claversal, Lixacol, Mezavant, Salofalk
- Canada: Asacol, Pentasa, Salofalk, Mezavant
- India: Mesacol (available as tablets, suppositories, enema), VEGAZ-OD.
- Mexico: Salofalk
- Serbia: Salofalk
- Turkey: Salofalk, Pentasa, Asacol, Encolit
- Uruguay: Mesacron, Mesalazina
- Brazil: Mesalazina, Mesalazina Enema, Mesacol, Mesacol MNX, Asalit,
- Australia: Mesasal, Pentasa, Salofalk, Mezavant (all source PBS)
- Egypt: Pentasa, Salofalk
- Belgium: Dipentum (olsalazine), Pentasa, Colitofalk, Asacol, Mezavant
- The Netherlands: Salofalk, Pentasa, Asacol, Mezavant
Chron-asa 5, Pentasa, Pentasa Enema, Mesaneo
Dosing depends on the preparation used; in particular, slow-release tablets may have quite different drug delivery characteristics and are not interchangeable.
Preparations that lower stool pH (such as lactulose, a laxative) will possibly affect the binding of mesalazine in the bowel and will therefore reduce its efficacy.
References
- ↑ Kruis, W.; Schreiber, I.; Theuer, D.; Brandes, J. W.; Schütz, E.; Howaldt, S.; Krakamp, B.; Hämling, J.; Mönnikes, H.; Koop, I.; Stolte, M.; Pallant, D.; Ewald, U. (2001). "Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses". Gut 49 (6): 783–789. doi:10.1136/gut.49.6.783. PMC 1728533. PMID 11709512.
- ↑ Sandborn WJ, Feagan BG, Lichtenstein GR (October 2007). "Medical management of mild to moderate Crohn's disease: evidence-based treatment algorithms for induction and maintenance of remission". Alimentary Pharmacology & Therapeutics 26 (7): 987–1003. doi:10.1111/j.1365-2036.2007.03455.x. PMID 17877506. Retrieved 2009-12-20.
- 1 2 "mesalazine". PharmGKB.
- ↑ Lippencott's Illustrated Reviews: Pharmacology, 4th Ed. Finkel, Cubeddu and Clark
- ↑ Drugs & Therapy Properties 2003 Oct; Vol 19, No. 10
- ↑ "Lialda Side Effects & Safety Information". Shire US. October 2007. Retrieved 2008-01-07.
- ↑ "Desensitization after fever induced by mesalazine". December 2001. Retrieved 2001-12-24.
- ↑ "MedlinePlus: Mesalamine". April 2014. Retrieved 2014-05-12.
- Mehta, Dinesh K, ed. (March 2003). British National Formulary 45. London: Pharmaceutical Press. ISBN 0-85369-555-5.
- Sweetman, Sean C, ed. (November 2004). Martindale: The complete drug reference (34th ed.). London: Pharmaceutical Press. ISBN 0-85369-550-4.
External links
| ||||||||||||||||||||||||||||||