Levopimaric acid
 | |
| Names | |
|---|---|
| IUPAC name
[1R-(1a,4ab,4ba,10aa)]-1,2,3,4,4a,4b,5,9,10,10a-Decahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid | |
| Other names
13-isopropylpodocarpa-8(14),12-dien-15-oic acid; D6,8(14)-abietadienoic acid; l-pimaric acid; β-pimaric acid; l-sapietic acid | |
| Identifiers | |
| 79-54-9 | |
| ChemSpider | 191771 |
| Jmol interactive 3D | Image |
| PubChem | 25201105 |
| |
| |
| Properties | |
| C20H30O2 | |
| Molar mass | 302.46 g·mol−1 |
| Appearance | Orthorhombic crystals |
| Melting point | 150 °C (302 °F; 423 K) |
| Practically insoluble in water | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Levopimaric acid is an abietane-type of diterpene resin acid.[1] It is a major constituent of pine oleoresin with the chemical formula of C20H30O2. In general, the abietene types of diterpene resin acid have various biological activities, such as antibacterial, cardiovascular and antioxidant. About 18 to 25% of levopimaric acid is found in pine oleoresin.[2] The production of oleoresin by conifer species is an important component of the defense response against insect attack and fungal pathogen infection.[3]
Resin acids
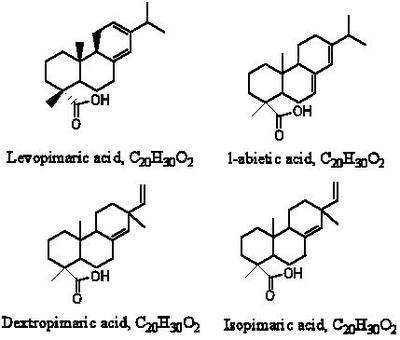
Resin acid is the general name for all kinds of acids that share the same basic skeleton, a three-fused ring and the empirical formula C20H30O2. The resin acids may be classified into two types, abietic and pimaric. The abietic-type group include levopimaric, l-abietic and neoabietic. The structure of these compounds differ only in the position of the conjugated double bond system. This feature influences their chemical reactivity. The pimaric-type acids are dextropimaric and isodextropimaric.[4]
Synthesis
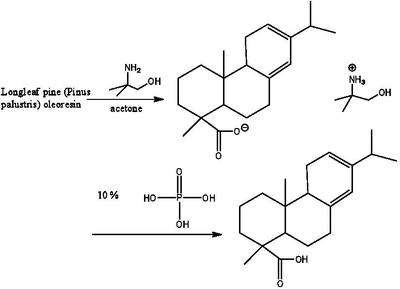
Levopimaric acid can be extracted by dissolving longleaf pine oleoresin in acetone and then adding 2-amino-2-methyl-1-propanol.[2]
Biosynthesis
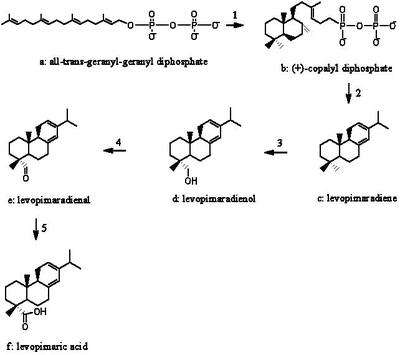
The abietane skeleton of levopimaric acid is formed by the cyclization of a diterpenoid precursor, substrate (a), all-trans-geranyl-geranyl diphophate (reaction 1). All diterpenes are considered to be derived from the C20 isoprenoid intermediate geranylgeranyl pyrophosphate.[5] The intermediate which is formed in Reaction (1), (+)-copalyl diphosphate, goes through the oxidation and rearrangement to give the intermediate (c), levopimaradiene, and diphosphate molecule. Then, the levopimaradiene goes through several more steps of oxidation processes, using oxygen molecules as the oxidizing agent, and NADPH as the proton donor.[6]
Role in biology
Oleoresin in pines is defined as pine gum, which is the nonaqueous secretion of resin acids dissolved in a terpene hydrocarbon oil, which is produced in or exuded from the intercellular resin ducts of a living tree. The viscous oleoresin secretion is composed of a complex mixture of terpenoids, consisting of roughly equal parts of volatile turpentine and rosin (also known as diterpene resin acids). Accumulated resin is released upon tissue injury and/or produced locally at the site of infestation, with the consequence that the beetle and associated fungal pathogens are killed, encased in resin, and expelled from the bore hole point of entry. This process is called pitching out, and it results in not only killing the attackers and flushing the wound site but also moving the oleoresin to the trunk surface where the turpentine evaporates to permit the resin acids to form a formidable physical barrier that seals the wound. Diterpene resin acids (DRA) play important roles in confer defense against insects and microbial pathogens. Levopimaric acid, an abietane-type DRA, is one of the principal resin acids.[3]
References
- ↑ Kersten, P. J., Kopper, B. J., Raffa, K. F., Illman, B. L. (2006). "Rapid Analysis of Abietanes in Conifers". J Chem Ecol 32 (12): 2679–2685. doi:10.1007/s10886-006-9191-z. PMID 17082986.
- 1 2 Lloyd, W. D.; Hedrick, G. W. (1965). "Levopimaric acid". Org. Synth. 45: 64.; Coll. Vol. 5, p. 699
- 1 2 Trapp, S.; Croteau, R. (2001). "Defensive Biosynthesis of Resin in Conifers". Annual Review of Plant Physiology and Plant Molecular Biology 52: 689–724. doi:10.1146/annurev.arplant.52.1.689. PMID 11337413.
- ↑ Baldwin, D., Loeblich, V., Lawrence, R. (1958). "Acidic Composition of Oleoresins and Rosins". Ind. Eng. Chem. Chem. Eng. Data Series 3 (2): 342–346. doi:10.1021/i460004a036.
- ↑ Mohamed Naceur Belgacem; Alessandro Gandini (3 June 2008). Monomers, polymers and composites from renewable resources. Elsevier. ISBN 978-0-08-045316-3. Retrieved 5 December 2011.
- ↑ LaFever, R. E., Vogel, B. S., Croteau, R. (1994). "Diterpenoid resin acid biosynthesis in conifers: enzymatic cyclization of geranylgeranyl pyrophosphate to abietadiene, the precursor of abietic acid". Arch Biochem Biophys 313 (1): 139–149. doi:10.1006/abbi.1994.1370. PMID 8053674.