Leuchs' anhydride
Leuchs' anhydride is an N-carboxyanhydride (NCA) first synthesized in 1906 by Hermann Leuchs, a German chemist.[1]
Synthesis
Leuchs' anhydride was first synthesized by reacting an N-ethoxycarbonyl or N-methoxycarbonyl amino acid chloride in a vacuum at 50-70 °C. Leuchs at al. prepared this reaction for the purpose of stepwise peptide synthesis. However, upon heating cyclization was observed and the reaction yielded NCAs and alkylchlorides.[2]

This synthesis of NCAs was deemed the Leuchs method. The relatively high temperatures necessary for this cyclization resulted in the decomposition of several NCAs. Therefore, Leuchs proposed several improvements in later years. One notable procedure involves reacting an unprotected amino acid with phosgene.[3]
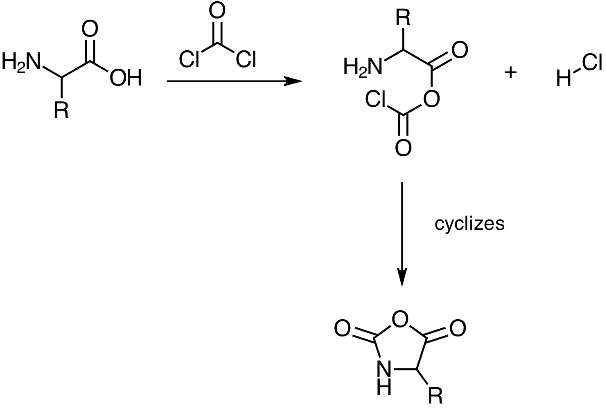
Leuchs' anhydride in peptide synthesis
Over one hundred years after its discovery, Leuchs' anhydride and variations thereof are still used for peptide synthesis. The NCA method allows rapid cyclization to produce the target peptide with high yield. Additionally, peptide synthesis reactions with NCAs don’t require protection of the amino acid functional groups. One downfall is the due to the high reactibility of NCAs, a small amount of by-product is sometimes formed. N-substituted NCAs can be used to make the by-products easier to remove.[4]
Leuchs' anhydride is also useful for the synthesis of amino acid polymers. Ephraim Katzir first used this method to synthesize poly-L-lysine by polymerizing N-carbobenzyloxy-α-N-carboxy-L-lysine anhydride and removing the carbobenzyloxy protecting group with phosphonium iodide.[5]
Leuchs' anhydride in siRNA delivery platform synthesis
One of the major problem encountered by siRNA -based therapeutic techniques because of its large size and negative charge density is that it lacks sufficient in-vivo delivery of the siRNA into the target cells. The most efficient way for the transfer of siRNA to the liver has been achieved by lipid nanoparticles, polymer conjugates and peptide conjugates. One of the majot drawback of current methods of the transfer is the biodegradability of the vessel once it is inside the body. Alternatively, Poly(amide) polymers can be synthesized using a facile N-carboxy anhydride (Leuchs' anhydride) (NCA) polymerization. The synthesis scheme of boc-l-ornithine N-carboxyanhydride is shown in the figure below. [6]
The monomer thus synthesized was then dissolved in anhydrous DMA (Dimethoxyamphetamine). N-butylamine and Phenylalanine-N-carboanhydride was then added to the reaction flask sequentially and allowed to react for 24 hrs and 8h respectively. The polymer was formed as precipitate and was dried. TFA was used to deprotect the polymer and was recovered as salt. [6]
The poly(amide) polymer reduces toxicity induced by biodegradibility and targets GaINAc (N-acetylgalactosamine: targeting ligands for hepatic specific uptake), pH dependent exposure of ionic groups to mediate endosomal exchange along with sharing similarities with other siRNA system. [6]
Imine-Leuchs' anhydride condensation
Leuchs' Anhydride has been recently used for the synthesis of Imidazolidin-4-ones. Imidazolin-4-ones are of high interest for synthetic and pharmaceutical industry owing to its antibacterial property and as a treatment for chronic inflammatory diseases such as multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease. A one-step synthesis of imidazolin-4-ones (which was not discovered till date) involves a tandem imine acyl substitution-decarboxylation followed by intramalecualr amide-imonium cyclization. The product formed is an imidazolin-4-one which can be either isolated as a diastereomers or be used to give a Schiff base via ring opening.
http://www.sciencedirect.com/science/article/pii/S0040403915006164
This method of synthesizing imidazolin-4-ones also has the advantage over other methodologies as it can be used to form an alpha amino acid deravitives under mild reaction condition.[7]
References
- ↑ Leuchs, Hermann (1906). "Ueber die Glycin-carbonsäure". Berichte der deutschen chemischen 39 (1): 857–861. doi:10.1002/cber.190603901133. ISSN 0365-9496.
- ↑ Kricheldorf, Hans Rytger (1987-01-01). Synthesis and Characterization of NCAs. Springer Berlin Heidelberg. pp. 3–58. ISBN 978-3-642-71588-4. Retrieved 25 December 2014.
- ↑ Montalbetti; et al. (August 2005). "Amide bond formation and peptide coupling" (PDF). Tetrahedron 61 (740): 10827–10852. doi:10.1016/j.tet.2005.08.031.
- ↑ Katakai, Ryoichi (September 1975). "Peptide synthesis using o-nitrophenylsulfenyl N-carboxy .alpha.-amino acid anhydrides". The Journal of Organic Chemistry 40 (19): 2697–2702. doi:10.1021/jo00907a001. Retrieved 25 December 2014.
- ↑ Katchalski-Katzir, Ephraim (February 2005). "My Contributions to Science and Society" (PDF). Journal of Biological Chemistry 280 (17): 16529–16541. doi:10.1074/jbc.X400013200. Retrieved 31 December 2014.
- 1 2 3 Barrett-, Stephanie.E. (June 2014). "Development of a liver-targeted siRNA delivery platform with a broad therapeutic window utilizing biodegradable polypeptide-based polymer conjugates,". Journal of Controlled Release 183: 124–137. doi:10.1016/j.jconrel.2014.03.028. Retrieved 19 December 2015.
- ↑ Sucu-Onur, Bilgesu (May 2015). "Direct synthesis of imidazolidin-4-ones via cycloadditions of imines with a Leuchs' anyhdride,". tetrahedron Letters 56 (20): 2590–2592. doi:10.1016/j.tetlet.2015.04.002. Retrieved 30 November 2015.