Laser-induced breakdown spectroscopy
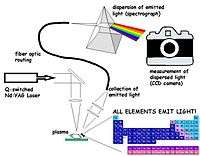
Laser-induced breakdown spectroscopy (LIBS) is a type of atomic emission spectroscopy which uses a highly energetic laser pulse as the excitation source.[1][2] The laser is focused to form a plasma, which atomizes and excites samples. In principle, LIBS can analyse any matter regardless of its physical state, be it solid, liquid or gas. Because all elements emit light of characteristic frequencies when excited to sufficiently high temperatures, LIBS can (in principle) detect all elements, limited only by the power of the laser as well as the sensitivity and wavelength range of the spectrograph & detector. If the constituents of a material to be analyzed are known, LIBS may be used to evaluate the relative abundance of each constituent element, or to monitor the presence of impurities. In practice, detection limits are a function of a) the plasma excitation temperature, b) the light collection window, and c) the line strength of the viewed transition. LIBS makes use of optical emission spectrometry and is to this extent very similar to arc/spark emission spectroscopy.
LIBS operates by focusing the laser onto a small area at the surface of the specimen; when the laser is discharged it ablates a very small amount of material, in the range of nanograms to picograms, which generates a plasma plume with temperatures in excess of 100,000 K. During data collection, typically after local thermodynamic equilibrium is established, plasma temperatures range from 5,000–20,000 K. At the high temperatures during the early plasma, the ablated material dissociates (breaks down) into excited ionic and atomic species. During this time, the plasma emits a continuum of radiation which does not contain any useful information about the species present, but within a very small timeframe the plasma expands at supersonic velocities and cools. At this point the characteristic atomic emission lines of the elements can be observed. The delay between the emission of continuum radiation and characteristic radiation is in the order of 10 µs, this is why it is necessary to temporally gate the detector.
LIBS can often be referred to as its alternative name: laser-induced plasma spectroscopy (LIPS). The term LIPS has alternative meanings that are outside the field of analytical spectroscopy, therefore the term LIBS is preferred.
LIBS is technically very similar to a number of other laser-based analytical techniques, sharing much of the same hardware. These techniques are the vibrational spectroscopic technique of Raman spectroscopy, and the fluorescence spectroscopic technique of laser-induced fluorescence (LIF). In fact devices are now being manufactured which combine these techniques in a single instrument, allowing the atomic, molecular and structural characterisation of a specimen as well as giving a deeper insight into physical properties.
Design
A typical LIBS system consists of a Nd:YAG solid-state laser and a spectrometer with a wide spectral range and a high sensitivity, fast response rate, time gated detector. This is coupled to a computer which can rapidly process and interpret the acquired data. As such LIBS is one of the most experimentally simple spectroscopic analytical techniques, making it one of the cheapest to purchase and to operate.
The Nd:YAG laser generates energy in the near infrared region of the electromagnetic spectrum, with a wavelength of 1064 nm. The pulse duration is in the region of 10 ns generating a power density which can exceed 1 GW·cm−2 at the focal point. Other lasers have been used for LIBS, mainly the Excimer (Excited dimer) type that generates energy in the visible and ultraviolet regions.
The spectrometer consists of either a monochromator (scanning) or a polychromator (non-scanning) and a photomultiplier or CCD detector respectively. The most common monochromator is the Czerny-Turner type whilst the most common polychromator is the Echelle type. However, even the Czerny-Turner type can be (and is often) used to disperse the radiation onto a CCD effectively making it a polychromator. The polychromator spectrometer is the type most commonly used in LIBS as it allows simultaneous acquisition of the entire wavelength range of interest.
The spectrometer collects electromagnetic radiation over the widest wavelength range possible, maximising the number of emission lines detected for each particular element. Spectrometer response is typically from 1100 nm (near infrared) to 170 nm (deep ultraviolet), the approximate response range of a CCD detector. All elements have emission lines within this wavelength range. The energy resolution of the spectrometer can also affect the quality of the LIBS measurement, since high resolution systems can separate spectral emission lines in close juxtaposition, reducing interference and increasing selectivity. This feature is particularly important in specimens which have a complex matrix, containing a large number of different elements. Accompanying the spectrometer and detector is a delay generator which accurately gates the detector's response time, allowing temporal resolution of the spectrum.
Advantages
Because such a small amount of material is consumed during the LIBS process the technique is considered essentially non-destructive or minimally-destructive, and with an average power density of less than one watt radiated onto the specimen there is almost no specimen heating surrounding the ablation site. Due to the nature of this technique sample preparation is typically minimised to homogenisation or is often unnecessary where heterogeneity is to be investigated or where a specimen is known to be sufficiently homogeneous, this reduces the possibility of contamination during chemical preparation steps. One of the major advantages of the LIBS technique is its ability to depth profile a specimen by repeatedly discharging the laser in the same position, effectively going deeper into the specimen with each shot. This can also be applied to the removal of surface contamination, where the laser is discharged a number of times prior to the analysing shot. LIBS is also a very rapid technique giving results within seconds, making it particularly useful for high volume analyses or on-line industrial monitoring.
LIBS is an entirely optical technique, therefore it requires only optical access to the specimen. This is of major significance as fiber optics can be employed for remote analyses. And being an optical technique it is non-invasive, non-contact and can even be used as a stand-off analytical technique when coupled to appropriate telescopic apparatus. These attributes have significance for use in areas from hazardous environments to space exploration. Additionally LIBS systems can easily be coupled to an optical microscope for micro-sampling adding a new dimension of analytical flexibility.
With specialised optics or a mechanically positioned specimen stage the laser can be scanned over the surface of the specimen allowing spatially resolved chemical analysis and the creation of 'elemental maps'. This is very significant as chemical imaging is becoming more important in all branches of science and technology.
Portable LIBS systems are more sensitive, faster and can detect a wider range of elements (particularly the light elements) than competing techniques such as portable x-ray fluorescence. And LIBS does not use ionizing radiation to excite the sample, which is both penetrating and potentially carcinogenic.
Disadvantages
LIBS, like all other analytical techniques is not without limitations. It is subject to variation in the laser spark and resultant plasma which often limits reproducibility. The accuracy of LIBS measurements is typically better than 10% and precision is often better than 5%. The detection limits for LIBS vary from one element to the next depending on the specimen type and the experimental apparatus used. Even so detection limits of 1 to 30 ppm by mass are not uncommon, but can range from >100 ppm to <1 ppm.
Recent developments
Recent interest in LIBS has focused on the miniaturization of the components and the development of compact, low-power, portable systems. Interest from groups such as NASA and ESA - as well as the military - has furthered these developments. The Mars Science Laboratory mission brought ChemCam, a LIBS instrument, to the surface of Mars in 2012.
Recent developments in LIBS have seen the introduction of double-pulsed laser systems.[3][4] For double-pulse LIBS one distinguishes between orthogonal and perpendicular configuration. In perpendicular configuration the laser fires twice on the same spot on the specimen with a pulse separation in the order of one to a couple of tens of microseconds. Depending on pulse separation, the second pulse is more or less absorbed by the plasma plume caused by the previous pulse, resulting in a reheating of the laser plasma leading to signal enhancement. In orthogonal configuration a laser pulse is fired parallel to the sample surface either before or after the perpendicular pulse hits the specimen. The laser plasma ignited in the surrounding medium above the surface by a first pulse causes (by its shock wave) an area of reduced pressure above the specimen into which the actual plasma from the sample can expand. This has similar positive effects on sensitivity like LIBS performed at reduced pressures. If the orthogonal laser pulse is delayed with respect to the perpendicular one, the effects are similar as in the perpendicular configuration. Timing electronics such as digital delay generators can precisely control the timing of both pulses.
Both double-pulse LIBS as well as LIBS at reduced pressures aim at increasing the sensitivity of LIBS and the reduction of errors caused by the differential volatility of elements (e.g. hydrogen as an impurity in solids). It also significantly reduces the matrix effects. Double-pulsed systems have proven useful in conducting analysis in liquids, as the initial laser pulse forms a cavity bubble in which the second pulse acts on the evaporated material.
LIBS is one of several analytical techniques that can be deployed in the field as opposed to pure laboratory techniques e.g. spark OES. As of 2015, recent research on LIBS focuses on compact and (man-)portable systems. Industrial applications of LIBS include (for example) the detection of material mix-ups, analysis of inclusions in steel, analysis of slags in secondary metallurgy and high-speed identification of scrap pieces for material-specific recycling tasks. Armed with data analysis techniques, this technique is being extended to pharmaceutical samples.[5]
LIBS using short laser pulses
Following multiphoton or tunnel ionization the electron is being accelerated by inverse Bremsstrahlung and can collide with the nearby molecules and generate new electrons through collisions. If the pulse duration is long, the newly ionized electrons can be accelerated and eventually avalanche or cascade ionization follows. Once the density of the electrons reaches a critical value, breakdown occurs and high density plasma is created which has no memory of the laser pulse. So, the criterion for the shortness of a pulse in dense media is as follows: A pulse interacting with a dense matter is considered to be short if during the interaction the threshold for the avalanche ionization is not reached. At the first glance this definition may appear to be too limiting. Fortunately, due to the delicately balanced behavior of the pulses in dense media, the threshold cannot be reached easily.[6] The phenomenon responsible for the balance is the intensity clamping[7] through the onset of filamentation process during the propagation of strong laser pulses in dense media.
A potentially important development to LIBS involves the use of a short laser pulse as a spectroscopic source.[8] In this method, a plasma column is created as a result of focusing ultrafast laser pulses in a gas. The self-luminous plasma is far superior in terms of low level of continuum and also smaller line broadening. This is attributed to the lower density of the plasma in the case of short laser pulses due to the defocusing effects which limits the intensity of the pulse in the interaction region and thus prevents further multiphoton/tunnel ionization of the gas.[9][10]
See also
- Spectroscopy
- Atomic spectroscopy
- Raman spectroscopy
- Laser-induced fluorescence
- List of plasma (physics) articles
- List of surface analysis methods
- Laser ablation
References
- ↑ Radziemski, Leon J.; Cremers, David A. (2006). Handbook of laser-induced breakdown spectroscopy. New York: John Wiley. ISBN 0-470-09299-8.
- ↑ Schechter, Israel; Miziolek, Andrzej W.; Vincenzo Palleschi (2006). Laser-induced breakdown spectroscopy (LIBS): fundamentals and applications. Cambridge, UK: Cambridge University Press. ISBN 0-521-85274-9.
- ↑ Ahmed, Rizwan; Baig, M. Aslam (2009). "A comparative study of single and double pulse laser induced breakdown spectroscopy". Journal of Applied Physics 106 (3): 033307. doi:10.1063/1.3190516. ISSN 0021-8979.
- ↑ Ahmed, R; Baig, M A (2010). "On the Optimization for Enhanced Dual-Pulse Laser-Induced Breakdown Spectroscopy". IEEE Transactions on Plasma Science 38 (8): 2052–2055. doi:10.1109/TPS.2010.2050784. ISSN 0093-3813.
- ↑ "Laser-induced breakdown spectroscopy-based investigation and classification of pharmaceutical tablets using multivariate chemometric analysis". doi:10.1016/j.talanta.2011.09.040.
- ↑ S. Mehdi Sharifi and Abdossamad Talebpour, Applications of Short Laser Pulses, cdn.intechopen.com
- ↑ Xu, Shengqi, et al. "Simple method of measuring laser peak intensity inside a femtosecond laser filament in air." Opt. Express 20.1 (2012): 299-307.
- ↑ A. Talebpour et al., Spectroscopy of the Gases Interactingwith Intense Femtosecond Laser Pulses, 2001, Laser Physics, 11:68–76
- ↑ A. Talebpour et al., Focusing limits of intense ultrafast laser pulses in a high pressure gas: road to new spectroscopic source, 2000, Optics Communications, 183:479–484
- ↑ Geints, Y. E., & Zemlyanov, A. A. (2009). On the focusing limit of high-power femtosecond laser pulse propagation in air. The European Physical Journal D,55(3), 745-754.
- Lee, Won‐Bae; Wu, Jianyong; Lee, Yong‐Ill; Sneddon, Joseph (2004). "Recent Applications of Laser‐Induced Breakdown Spectrometry: A Review of Material Approaches". Applied Spectroscopy Reviews 39 (1): 27–97. doi:10.1081/ASR-120028868. ISSN 0570-4928.
- Noll, Reinhard; Bette, Holger; Brysch, Adriane; Kraushaar, Marc; Mönch, Ingo; Peter, Laszlo; Sturm, Volker (2001). "Laser-induced breakdown spectrometry — applications for production control and quality assurance in the steel industry". Spectrochimica Acta Part B: Atomic Spectroscopy 56 (6): 637–649. doi:10.1016/S0584-8547(01)00214-2. ISSN 0584-8547.
Further reading
- Andrzej W. Miziolek, Vincenzo Palleschi, Israel Schechter (2006). Laser Induced Breakdown Spectroscopy. New York: Cambridge University Press. ISBN 0-521-85274-9.
- Gornushkin, I.B.; Amponsah-Manager, K.; Smith, B.W.; Omenetto, N.; Winefordner, J.D. (2004). "Microchip Laser Induced Breakdown Spectroscopy: Preliminary Feasibility Investigation". Applied Spectroscopy 58 (7): 762–769. doi:10.1366/0003702041389427. PMID 15282039.
- Amponsah-Manager, K.; Omenetto, N.; Smith, B. W.; Gornushkin, I. B.; Winefordner, J. D. (2005). "Microchip laser ablation of metals: Investigation of the ablation process in view of its application to laser-induced breakdown spectroscopy". Journal of Analytical Atomic Spectrometry 20 (6): 544. doi:10.1039/B419109A.
- Lopez-Moreno, C.; Amponsah-Manager, K.; Smith, B. W.; Gornushkin, I. B.; Omenetto, N.; Palanco, S.; Laserna, J. J.; Winefordner, J. D. (2005). "Quantitative analysis of low-alloy steel by microchip laser induced breakdown spectroscopy". Journal of Analytical Atomic Spectrometry 20 (6): 552. doi:10.1039/B419173K.
- Bette, H; Noll, R (2004). "High speed laser-induced breakdown spectrometry for scanning microanalysis". Journal of Physics D: Applied Physics 37 (8): 1281. doi:10.1088/0022-3727/37/8/018.
- Balzer, Herbert; Hoehne, Manuela; Noll, Reinhard; Sturm, Volker (2006). "New approach to online monitoring of the Al depth profile of the hot-dip galvanised sheet steel using LIBS". Analytical and Bioanalytical Chemistry 385 (2): 225–33. doi:10.1007/s00216-006-0347-z. PMID 16570144.
- Sturm, V.; Peter, L.; Noll, R. (2000). "Steel analysis with laser-induced breakdown spectrometry in the vacuum ultraviolet". Appl. Spectroscopy 54 (9): 1275–1278. doi:10.1366/0003702001951183.
- Vadillo, José M.; Laserna, J.Javier (2004). "Laser-induced plasma spectrometry: Truly a surface analytical tool". Spectrochimica Acta Part B: Atomic Spectroscopy 59 (2): 147. doi:10.1016/j.sab.2003.11.006.
- Doucet, François R.; Faustino, Patrick J.; Sabsabi, Mohamad; Lyon, Robbe C. (2008). "Quantitative molecular analysis with molecular bands emission using laser-induced breakdown spectroscopy and chemometrics". Journal of Analytical Atomic Spectrometry 23 (5): 694. doi:10.1039/b714219f.
- В.Копачевский, В.Шпектор, Д.Клемято, В.Бойков, М.Кривошеева, Л.Боброва. (2008). "Количественный анализ состава тарных стекол анализатором LEA S500". Фотоника (in Russian) (1): 38–40.
- Noll, Reinhard (2012). Laser-Induced Breakdown Spectroscopy: Fundamentals and Applications. Berlin: Springer. ISBN 3-642-20667-0.
| ||||||||||||||||||||||||||||||||||||||||||||||