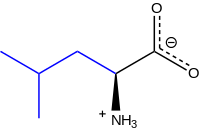
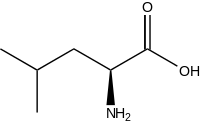
Leucine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Leucine | |
| Other names
2-Amino-4-methylpentanoic acid | |
| Identifiers | |
| 61-90-5 | |
| ChEBI | CHEBI:57427 |
| ChEMBL | ChEMBL291962 |
| ChemSpider | 5880 |
| DrugBank | DB01746 |
| 3312 | |
| Jmol interactive 3D | Image |
| KEGG | D00030 |
| PubChem | 6106 |
| UNII | GMW67QNF9C |
| |
| |
| Properties | |
| C6H13NO2 | |
| Molar mass | 131.18 g·mol−1 |
| Acidity (pKa) | 2.36 (carboxyl), 9.60 (amino)[1] |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| | |
| Infobox references | |
Leucine (abbreviated as Leu or L; encoded by the six codons UUA, UUG, CUU, CUC, CUA, and CUG) is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated -+NH3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated –COO- form under biological conditions), and an isobutyl side chain, classifying it as a nonpolar (at physiological pH) amino acid. It is essential in humans, meaning the body cannot synthesize it and thus it must be obtained from the diet.
Leucine is a major component of the subunits in ferritin, astacin, and other "buffer" proteins.
Biology
Leucine is utilized in the liver, adipose tissue, and muscle tissue. In adipose and muscle tissue, leucine is used in the formation of sterols, and the combined usage of leucine in these two tissues is seven times greater than its use in the liver.[2]
Biosynthesis
As an essential amino acid, leucine cannot be synthesized by animals. Consequently, it must be ingested, usually as a component of proteins. In plants and microorganisms, leucine is synthesised from pyruvic acid by a series of enzymes:[3]
- Acetolactate synthase
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- α-Isopropylmalate synthase
- α-Isopropylmalate isomerase
- Leucine aminotransferase
Synthesis of the small, hydrophobic amino acid valine also includes the initial part of this pathway.
Effects
Leucine is an activator of mTOR; it is the only dietary amino acid that has the capacity to directly stimulate muscle protein synthesis.[4] As a dietary supplement, leucine has been found to slow the degradation of muscle tissue by increasing the synthesis of muscle proteins in aged rats.[5] However, results of comparative studies are conflicted. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men.[6] More studies are needed, preferably those which utilize an objective, random sample of society. Factors such as lifestyle choices, age, gender, diet, exercise, etc. must be factored into the analyses in order to isolate the effects of supplemental leucine as a standalone, or if taken with other branched chain amino acids (BCAAs). Until then, dietary supplemental leucine cannot be associated as the prime reason for muscular growth or optimal maintenance for the entire population.
Leucine potently activates the mammalian target of rapamycin kinase that regulates cell growth. Infusion of leucine into the rat brain has been shown to decrease food intake and body weight via activation of the mTOR pathway.[7]
Both L-leucine and D-leucine protect mice against seizures.[8] D-leucine also terminates seizures in mice after the onset of seizure activity, at least as effectively as diazepam and without sedative effects.[8]
Safety
Leucine toxicity, as seen in decompensated maple syrup urine disease (MSUD), causes delirium and neurologic compromise, and can be life-threatening.
Excess leucine may be a cause of pellagra, whose main symptoms are "the four D's": diarrhea, dermatitis, dementia and death,[9] though the relationship is unclear.[10]
Leucine at a dose exceeding 500 mg/kg/d was observed with hyperammonemia.[11] As such, the UL for leucine in healthy adult men can be suggested at 500 mg/kg/d or 35 g/d under acute dietary conditions.[11]
Dietary sources
| Food | g/100g |
|---|---|
| Soybeans, mature seeds, raw | 2.97 |
| Beef, round, top round, separable lean and fat, trimmed to 3 mm (1⁄8 in) fat, select, raw | 1.76 |
| Peanuts | 1.672 |
| Salami, pork | 1.63 |
| Fish, salmon, pink, raw | 1.62 |
| Wheat germ | 1.571 |
| Almonds | 1.488 |
| Chicken, broilers or fryers, thigh, meat only, raw | 1.48 |
| Chicken egg, yolk, raw, fresh | 1.40 |
| Oat | 1.284 |
| Beans, pinto, cooked | 0.765 |
| Lentils, cooked | 0.654 |
| Chickpea, cooked | 0.631 |
| Corn, yellow | 0.348 |
| Cow milk, whole, 3.25% milk fat | 0.27 |
| Rice, brown, medium-grain, cooked | 0.191 |
| Milk, human, mature, fluid | 0.10 |
Chemical properties

Leucine is a branched-chain amino acid (BCAA) since it possesses an aliphatic side-chain that is non-linear.
Racemic leucine had been subjected to circularly polarized synchrotron radiation in order to better understand the origin of biomolecular asymmetry. An enantiomeric enhancement of 2.6% had been induced, indicating a possible photochemical origin of biomolecules' homochirality.[13]
Other uses
As a food additive, L-leucine has E number E641 and is classified as a flavor enhancer.[14]
See also
- Leucines, the isomers and derivatives of leucine
References
- ↑ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ↑ J. Rosenthal, et al. Department of Medicine, University of Toronto, Toronto, Canada. "Metabolic fate of leucine: A significant sterol precursor in adipose tissue and muscle". American Journal of Physiology Vol. 226, No. 2, p. 411-418. Retrieved 2008-03-25.
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Etzel MR (2004). "Manufacture and use of dairy protein fractions". The Journal of Nutrition 134 (4): 996S–1002S. PMID 15051860.
- ↑ L. Combaret, et al. Human Nutrition Research Centre of Clermont-Ferrand. "A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle". Journal of Physiology Volume 569, issue 2, p. 489-499. Retrieved 2008-03-25.
- ↑ Am J Clin Nutr. (2009 issue=May 89(5)). "Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men". Am J Clin Nutr 89 (5): 1468–75. doi:10.3945/ajcn.2008.26668. PMID 19321567. Check date values in:
|date=(help) - ↑ Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ (2006). "Hypothalamic mTOR signaling regulates food intake". Science 312 (5775): 927–930. doi:10.1126/science.1124147. PMID 16690869.
- 1 2 Hartman AL, Santos P, O'Riordan KJ, Stafstrom CE, Marie Hardwick J (2015). "Potent anti-seizure effects of D-leucine". Neurobiology of Disease 82: 46–53. doi:10.1016/j.nbd.2015.05.013. PMID 26054437. Retrieved 2015-11-26.
- ↑ Hegyi J, Schwartz R, Hegyi V (2004). "Pellagra: dermatitis, dementia, and diarrhea". Int J Dermatol 43 (1): 1–5. doi:10.1111/j.1365-4632.2004.01959.x. PMID 14693013.
- ↑ Bapurao S, Krishnaswamy K (1978). "Vitamin B6 nutritional status of pellagrins and their leucine tolerance". Am J Clin Nutr 31 (5): 819–24. PMID 206127.
- 1 2 Elango R, Chapman K, Rafii M, Ball RO, Pencharz PB (2012). "Determination of the tolerable upper intake level of leucine in acute dietary studies in young men". The American Journal of Clinical Nutrition 96 (4): 759–67. doi:10.3945/ajcn.111.024471. PMID 22952178. Retrieved 2015-12-07.
A significant increase in blood ammonia concentrations above normal values, plasma leucine concentrations, and urinary leucine excretion were observed with leucine intakes >500 mg · kg⁻¹ · d⁻¹. The oxidation of l-[1-¹³C]-leucine expressed as label tracer oxidation in breath (F¹³CO₂), leucine oxidation, and α-ketoisocaproic acid (KIC) oxidation led to different results: a plateau in F¹³CO₂ observed after 500 mg · kg⁻¹ · d⁻¹, no clear plateau observed in leucine oxidation, and KIC oxidation appearing to plateau after 750 mg · kg⁻¹ · d⁻¹. On the basis of plasma and urinary variables, the UL for leucine in healthy adult men can be suggested at 500 mg · kg⁻¹ · d⁻¹ or ~35 g/d as a cautious estimate under acute dietary conditions.
- ↑ National Nutrient Database for Standard Reference. U.S. Department of Agriculture. Retrieved 2009-09-16.
- ↑ Meierhenrich: Amino acids and the asymmetry of life, Springer-Verlag, 2008, ISBN 978-3-540-76885-2.
- ↑ Winter, Ruth (2009). A consumer's dictionary of food additives (7th ed.). New York: Three Rivers Press. ISBN 0307408922.
External links
- Leucine biosynthesis
- Leucine prevents muscle loss in rats
- Leucine helps regulate appetite in rats
- Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects
- A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
