Carnitine
 | |
| Systematic (IUPAC) name | |
|---|---|
|
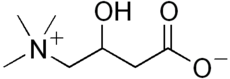
3-Hydroxy-4-(trimethylazaniumyl)butanoate | |
| Clinical data | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral, intravenous |
| Pharmacokinetic data | |
| Bioavailability | < 10% |
| Protein binding | None |
| Metabolism | slightly |
| Excretion | Urine (> 95%) |
| Identifiers | |
| CAS Number |
541-15-1 |
| ATC code | A16AA01 (L form) |
| PubChem | CID 288 |
| DrugBank |
DB00583 |
| ChemSpider |
282 |
| UNII |
0G389FZZ9M |
| KEGG |
C00318 |
| ChEBI |
CHEBI:17126 |
| ChEMBL |
CHEMBL172513 |
| Chemical data | |
| Formula | C7H15NO3 |
| Molar mass | 161.199 g/mol |
| |
| |
| | |
Carnitine is an amino acid derivative and nutrient involved in lipide (fat) metabolism in mammals and other eukaryotes. It is in the chemical compound classes of β-hydroxyacids and quaternary ammonium compounds, and because of the hydroxyl-substituent, it exists in two stereoisomers, the biologically active enantiomer L-carnitine, and the essentially biologically inactive D-carnitine.[1] Both are available through chemical synthesis, and the L-form is continuously biosynthesized in eukaryotic organisms from the proteinogenic amino acids lysine and methionine.[2] In such eukaryotic cells, it is specifically required for the transport of fatty acids from the intermembraneous space in the mitochondria into the mitochondrial matrix during the catabolism of lipids, in the generation of metabolic energy.[1] Carnitine was originally found as a growth factor for mealworms and labeled vitamin BT, although carnitine is not by biochemical definition a true vitamin.[3][4] It is used efficaciously, clinically, in the treatment of some conditions, e.g. systemic primary carnitine deficiency,[5] and it is available over the counter as a nutritional supplement, though its efficacy for most conditions for which it is advertised is controversial or not yet established.[6][7]
Biosynthesis and metabolism
In animals, the biosynthesis of carnitine occurs primarily in the liver and kidneys from the amino acids lysine (via trimethyllysine) and methionine.[8]
Carnitine transports long-chain acyl groups from fatty acids into the mitochondrial matrix, so they can be broken down through β-oxidation to acetyl CoA to obtain usable energy via the citric acid cycle.[1] In some organisms, such as fungi, the acetate is used in the glyoxylate cycle for gluconeogenesis and formation of carbohydrates. Fatty acids must be activated before being covalently linked to the carnitine molecule to form acylcarnitine for transport. The free fatty acid in the cytosol is first adenylated by reaction with ATP, then attached with a thioester bond to coenzyme A (CoA), with expulsion of AMP. This reaction is catalyzed by the enzyme fatty acyl-CoA synthetase and driven to completion by inorganic pyrophosphatase.
The acyl group on CoA can now be transferred to carnitine and the resulting acylcarnitine transported into the mitochondrial matrix.[1] This occurs via a series of similar steps:
- Acyl CoA is transferred to the hydroxyl group of carnitine by carnitine acyltransferase I (palmitoyltransferase) located on the outer mitochondrial membrane
- Acylcarnitine is shuttled inside by a carnitine-acylcarnitine translocase
- Acylcarnitine is converted to acyl CoA by carnitine acyltransferase II (palmitoyltransferase) located on the inner mitochondrial membrane. The liberated carnitine returns to the cytosol.
Carnitine acyltransferase I and peroxisomal carnitine octanoyl transferase (CROT) undergo allosteric inhibition by malonyl-CoA, an intermediate in fatty acid biosynthesis, to prevent futile cycling between β-oxidation and fatty acid synthesis.
Human genetic disorders, such as primary carnitine deficiency, carnitine palmitoyltransferase I deficiency, carnitine palmitoyltransferase II deficiency and carnitine-acylcarnitine translocase deficiency, affect different steps of this process.[9]
Physiological effects
Atherosclerosis
A link between dietary consumption of carnitine and atherosclerosis has been proposed.[10] There is evidence that carnitine lowers the risk of mortality due to arrythmias, after an acute myocardial infarction. When intestinal bacteria are exposed to carnitine from food, they produce the byproduct, trimethylamine, which is oxidised in the liver to trimethylamine N-oxide (TMAO), which may be associated with atherosclerosis; the risk of cardiovascular events is higher in those with high TMAO levels, independent of the observed level of carnitine. The presence of TMAO-producing bacteria is reportedly a consequence of subject's consuming diets rich in meat. Vegetarian and vegans who ate a single meal of meat had much lower levels of TMAO in their bloodstream than did regular meat-eaters, which was ascribed to the lower levels of the intestinal bacteria that convert carnitine into TMAO.[11]
A further study reported evidence of a second path for atherogenic activity of carnitine, passing through a different metabolite, γ-butyrobetaine (γBB).[12]
Effects on bone mass
Carnitine concentration in cells diminish as humans age, affecting fatty acid metabolism in various tissues. Bone is affected, in particular, as it requires the continuous reconstructive and metabolic functions of osteoblasts for maintenance of its mass. A 2008 study reported that supplementing rat diets with L-carnitine decreased bone turnover and increased bone mineral density in ovariectomized females.[13]
Effect on thyroid hormone action
A 2001 report suggested that L-carnitine may be useful in preventing and reversing hyperthyroid symptoms.[14] A 2004 study reported that L-carnitine acts as a peripheral antagonist of thyroid hormone action; in particular, L-carnitine inhibited both triiodothyronine (T3) and thyroxine (T4) entry into the cell nuclei.[15]
Possible health effects
Carnitine has been proposed as a supplement to treat a variety of health conditions including heart attack,[16][17] heart failure, angina,[18] narcolepsy,[19] and diabetic neuropathy,[20] but not improving exercise performance,[18] nor wasting syndrome (weight loss).[20] In all of these cases the results are preliminary or proposed, and not part of an established medical treatment.[20]
Sources
Food
The highest concentrations of carnitine are found in red meat. It can be found at significantly lower levels in many other foods including nuts and seeds (e.g. pumpkin, sunflower, sesame), legumes or pulses (beans, peas, lentils, peanuts), vegetables (artichokes, asparagus, beet greens (young leaves of the beetroot), broccoli, brussels sprouts, collard greens, garlic, mustard greens, okra, parsley, kale), fruits (apricots, bananas), cereals (buckwheat, corn, millet, oatmeal, rice bran, rye, whole wheat, wheat bran, wheat germ) and other foods (bee pollen, brewer's yeast, carob).
| Product | Quantity | Carnitine |
|---|---|---|
| Lamb | 100 g | 190 mg |
| Beef steak | 100 g | 95 mg |
| Ground beef | 100 g | 94 mg |
| Pork | 100 g | 27.7 mg |
| Bacon | 100 g | 23.3 mg |
| Tempeh | 100 g | 19.5 mg |
| Cod fish | 100 g | 5.6 mg |
| Chicken breast | 100 g | 3.9 mg |
| American cheese | 100 g | 3.7 mg |
| Ice cream | 100 ml | 3.7 mg |
| Whole milk | 100 ml | 3.3 mg |
| Avocado | one medium | 2 mg[21] |
| Cottage cheese | 100 g | 1.1 mg |
| Whole-wheat bread | 100 g | 0.36 mg |
| Asparagus | 100 g | 0.195 mg |
| White bread | 100 g | 0.147 mg |
| Macaroni | 100 g | 0.126 mg |
| Peanut butter | 100 g | 0.083 mg |
| Rice (cooked) | 100 g | 0.0449 mg |
| Egg | 100 g | 0.0121 mg |
| Orange juice | 100 ml | 0.0019 mg |
| Lentil | 100 g | 2.1 mg[22] |
| Potato | 100 g | 2.4 mg[22] |
| Sweet Potato | 100 g | 1.1 mg[22] |
| Banana | 100 g | 0.2 mg[22] |
| Carrot | 100 g | 0.3 mg[22] |
| Apple (without skin) | 100 g | 0.2 mg[22] |
| Raisin | 100 g | 0.8 mg[22] |
In general, 20 to 200 mg are ingested per day by those on an omnivorous diet, whereas those on a strict vegetarian or vegan diet may ingest as little as 1 mg/day. However, even strict vegetarians (vegans) show no signs of carnitine deficiency, despite the fact that most dietary carnitine is derived from animal sources.[23][24] No advantage appears to exist in giving an oral dose greater than 2 g at one time, since absorption studies indicate saturation at this dose.[25]
Health Canada
Other sources may be found in over-the-counter vitamins, energy drinks and various other products. Products containing L-carnitine can now be marketed as "natural health products" in Canada. As of 2012, Parliament has allowed carnitine products and supplements to be imported into Canada (Health Canada). The Canadian government did issue an amendment in December 2011 allowing the sale of L-carnitine without a prescription.[26]
See also
References
- 1 2 3 4 Mehta, Sweety (2013). [no editor], ed. "Activation and Transportation of Fatty Acids to the Mitochondria via the Carnitine Shuttle with Animation [self-published learning resource]" (online).
Disclaimer: PharmaXChange.info does not evaluate or guarantee the accuracy of any articles, videos or other posted information on the PharmaXChange.info Author Network (“PAN”)… see .
- ↑ Steiber A., J. Kerner & C. Hoppel (2004). "Carnitine: a Nutritional, Biosynthetic, and Functional perspective". Mol. Aspects Med. 25 (5–6): 455–73. doi:10.1016/j.mam.2004.06.006. PMID 15363636. Retrieved 22 January 2016.
- ↑ Bremer, J. (1983). "Carnitine—Metabolism and Functions". Physiol. Rev. 63: 1420–1480. Retrieved 22 January 2016.
- ↑ Carter, H. E.; Bhattacharyya, P. K.; Weidman, K. R.; Fraenkel, G. (1952). "Chemical Studies on vitamin BT. Isolation and characterization as carnitine". Arch. Biochem. Biophys 38: 405–416.
- ↑ Stanley, Charles A.; Bennett, Michael J.; Longo, Nicolo (2000). "Plasma Membrane Carnitine Transport Defect". In Scriver, C.W.; Beaudet, A.L.; Sly, W.S.; Valle, D. Metabolic and Molecular Bases of Inherited Disease (8th ed.). New York, NY, USA: McGraw Hill. doi:10.1036/ommbid.297. ISBN 0079130356. Retrieved 22 January 2016.
- ↑ Johri, A.M., D.K. Heyland, M.F. Hétu, B. Crawford & J.D. Spence (2014). "Carnitine Therapy for the Treatment of Metabolic Syndrome and Cardiovascular Disease: Evidence and Controversies" (print, online review). Nutr. Metab. Cardiovasc. Dis. 24 (8, Aug.): 808–814. doi:10.1016/j.numecd.2014.03.007. Retrieved 22 January 2016.
- ↑ Dambrova, M. & E. Liepinsh (2015). "Risks and Benefits of Carnitine Supplementation in Diabetes" (print, online review). Exp. Clin. Endocrinol. Diabetes 123 (2, Feb.): 95–100. doi:10.1055/s-0034-1390481. Retrieved 22 January 2016.
- ↑ "L-Carnitine". Archived from the original on 2007-05-08. Retrieved 2007-06-01.
- ↑ Olpin S (2005). "Fatty acid oxidation defects as a cause of neuromyopathic disease in infants and adults". Clin. Lab. 51 (5–6): 289–306. PMID 15991803.
- ↑ Brown, J. Mark & Stanley L. Hazen (2015). "The Gut Microbial Endocrine Organ: Bacterially Derived Signals Driving Cardiometabolic Diseases." (book chapter, review). Annu. Rev Med. 66: 343–359. doi:10.1146/annurev-med-060513-093205. Retrieved 22 January 2016.
- ↑ Koeth, Robert A., Zeneng Wang, Bruce S. Levison, Jennifer A. Buffa, Elin Org, Brendan T. Sheehy, Earl B. Britt, Xiaoming Fu, Yuping Wu, Lin Li, Jonathan D. Smith, Joseph A. DiDonato, Jun Chen, Hongzhe Li, Gary D. Wu, James D. Lewis, Manya Warrier, J. Mark Brown, Ronald M. Krauss, W. H. Wilson Tang, Frederic D. Bushman, Aldons J. Lusis, & Stanley L. Hazen (2013). "Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis". Nature Medicine 19 (5): 576–85. doi:10.1038/nm.3145. PMC 3650111. PMID 23563705. Retrieved 22 January 2016.
- ↑ Koeth, Robert A., Bruce S. Levison, Miranda K. Culley, Jennifer A. Buffa, Zeneng Wang, Jill C. Gregory, Elin Org, Yuping Wu, Lin Li, Jonathan D. Smith, W.H. Wilson Tang, Joseph A. DiDonato, Aldons J. Lusis, Stanley L. Hazen (2014). "γ-Butyrobetaine Is a Proatherogenic Intermediate in Gut Microbial Metabolism of L-Carnitine to TMAO". Cell Metabolism 20 (5): 799–812. doi:10.1016/j.cmet.2014.10.006. Retrieved 22 January 2016.
- ↑ Hooshmand S, Balakrishnan A, Clark RM, Owen KQ, Koo SI, Arjmandi BH (Aug 2008). "Dietary L-Carnitine Supplementation Improves Bone Mineral Density by Suppressing Bone Turnover in Aged Ovariectomized Rats". Phytomedicine 15: 595–601. doi:10.1016/j.phymed.2008.02.026.
- ↑ Benvenga S1, Ruggeri RM, Russo A, Lapa D, Campenni A, Trimarchi F (Aug 2001). "Usefulness of L-carnitine, a Naturally Occurring Peripheral Antagonist of Thyroid Hormone Action, in Iatrogenic Hyperthyroidism: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial". The Journal of Clinical Endocrinology and Metabolism 86: 3579–94. doi:10.1210/jcem.86.8.7747.
- ↑ Benvenga S, Amato A, Calvani M, Trimarchi F (Nov 2004). "Effects of carnitine on thyroid hormone action". Ann N Y Acad Sci 1033: 158–167. doi:10.1196/annals.1320.015. PMID 15591013.
- ↑ Dinicolantonio, J. J.; Lavie, C. J.; Fares, H.; Menezes, A. R.; o’Keefe, J. H. (2013). "L-Carnitine in the Secondary Prevention of Cardiovascular Disease: Systematic Review and Meta-analysis" (pdf). Mayo Clinic Proceedings 88 (6): 544–51. doi:10.1016/j.mayocp.2013.02.007. PMID 23597877.
- ↑ Marcovina, S. M.; Sirtori, C.; Peracino, A.; Gheorghiade, M.; Borum, P.; Remuzzi, G.; Ardehali, H. (2013). "Translating the Basic Knowledge of Mitochondrial Functions to Metabolic Therapy: Role of L-Carnitine". Translational Research 161 (2): 73–84. doi:10.1016/j.trsl.2012.10.006. PMC 3590819. PMID 23138103.
- 1 2 Pekala, J.; Patkowska-Sokoła, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochyński, S.; Librowski, T. (2011). "L-Carnitine—Metabolic Functions and Meaning in Humans [sic.] Life". Current Drug Metabolism 12 (7): 667–678. doi:10.2174/138920011796504536. PMID 21561431.
- ↑ Miyagawa, T; Kawamura, H; Obuchi, M; Ikesaki, A; Ozaki, A; Tokunaga, K; Inoue, Y; Honda, M (2013). "Effects of oral L-carnitine administration in narcolepsy patients: A randomized, double-blind, cross-over and placebo-controlled trial". PLoS ONE 8 (1): e53707. doi:10.1371/journal.pone.0053707. PMC 3547955. PMID 23349733.
- 1 2 3 Ehrlich, Steven D. (2014). "Supplement: Carnitine (L-carnitine)" (online). Complementary and Alternative Medicine Guide (December 28). Retrieved 21 January 2016.
[Author] Steven D. Ehrlich, NMD [Naturopathic Medical Doctor], Solutions Acupuncture, a private practice specializing in complementary and alternative medicine, Phoenix, AZ. Review provided by VeriMed Healthcare Network.
See Naturopathy. - ↑ Rebouche CJ. Carnitine. In: Shils ME, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease. 9th ed. Philadelphia: Lippincott, Williams & Wilkins; 1999:505-512. Cited by Jane Higdon (2002), see , accessed 12 January 2016
- 1 2 3 4 5 6 7 Demarquoy, Jean; Georges, Béatrice; Rigault, Caroline; Royer, Marie-Charlotte; Clairet, Amélie; Soty, Maud; Lekounoungou, Serge; Le Borgne, Françoise (2004-06-01). "Radioisotopic determination of l-carnitine content in foods commonly eaten in Western countries". Food Chemistry 86 (1): 137–142. doi:10.1016/j.foodchem.2003.09.023.
- ↑ http://lpi.oregonstate.edu/infocenter/othernuts/carnitine/
- ↑ Lombard, K. A.; Olson, A. L.; Nelson, S. E.; Rebouche, C. J. (1989-08-01). "Carnitine status of lactoovovegetarians and strict vegetarian adults and children". The American Journal of Clinical Nutrition 50 (2): 301–306. ISSN 0002-9165. PMID 2756917.
- ↑ Bain, Marcus A.; Milne, Robert W.; Evans, Allan M. (2006-10-01). "Disposition and metabolite kinetics of oral L-carnitine in humans". Journal of Clinical Pharmacology 46 (10): 1163–1170. doi:10.1177/0091270006292851. ISSN 0091-2700. PMID 16988205.
- ↑ "Regulations Amending the Food and Drug Regulations".
Further reading
The following are good secondary sources on the subject of this article.
- Bremer, J (1983). "Carnitine—Metabolism and Functions". Physiol. Rev. 63: 1420–1480. Retrieved 22 January 2016.
- Stanley, Charles A.; Bennett, Michael J.; Longo, Nicolo (2000). "Plasma Membrane Carnitine Transport Defect". In Scriver, C.W.; Beaudet, A.L.; Sly, W.S.; Valle, D. Metabolic and Molecular Bases of Inherited Disease (8th ed.). New York, NY, USA: McGraw Hill. doi:10.1036/ommbid.297. ISBN 0079130356. Retrieved 22 January 2016.
- Steiber A., J. Kerner & C. Hoppel (2004). "Carnitine: a Nutritional, Biosynthetic, and Functional perspective". Mol. Aspects Med. 25 (5–6): 455–73. doi:10.1016/j.mam.2004.06.006. PMID 15363636. Retrieved 22 January 2016.
- Marcovina, S. M.; Sirtori, C.; Peracino, A.; Gheorghiade, M.; Borum, P.; Remuzzi, G.; Ardehali, H. (2013). "Translating the Basic Knowledge of Mitochondrial Functions to Metabolic Therapy: Role of L-Carnitine". Translational Research 161 (2): 73–84. doi:10.1016/j.trsl.2012.10.006. PMC 3590819. PMID 23138103.
- Johri, A.M., D.K. Heyland, M.F. Hétu, B. Crawford & J.D. Spence (2014). "Carnitine Therapy for the Treatment of Metabolic Syndrome and Cardiovascular Disease: Evidence and Controversies" (print, online review). Nutr. Metab. Cardiovasc. Dis. 24 (8, Aug.): 808–814. doi:10.1016/j.numecd.2014.03.007. Retrieved 22 January 2016.
- Dambrova, M. & E. Liepinsh (2015). "Risks and Benefits of Carnitine Supplementation in Diabetes" (print, online review). Exp. Clin. Endocrinol. Diabetes 123 (2, Feb.): 95–100. doi:10.1055/s-0034-1390481. Retrieved 22 January 2016.
- Brown, J. Mark & Stanley L. Hazen (2015). "The Gut Microbial Endocrine Organ: Bacterially Derived Signals Driving Cardiometabolic Diseases." (book chapter, review). Annu. Rev Med. 66: 343–359. doi:10.1146/annurev-med-060513-093205. Retrieved 22 January 2016.
External links
| ||||||||||||||
| ||||||||||||||
|