Autoignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature at which it will spontaneously ignite in normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to supply the activation energy needed for combustion. The temperature at which a chemical will ignite decreases as the pressure or oxygen concentration increases. It is usually applied to a combustible fuel mixture.
Autoignition temperatures of liquid chemicals are typically measured using a 500 mL flask placed in a temperature controlled oven in accordance with the procedure described in ASTM E659.[1]
When measured for plastics, autoignition temperature can also be measured under elevated pressure and at 100% oxygen concentration. The resulting value is used as a predictor of viability for high-oxygen service. The main testing standard for this is ASTM G72.[2]
Autoignition equation
The time 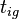 it takes for a material to reach its autoignition temperature
it takes for a material to reach its autoignition temperature 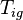 when exposed to a heat flux
when exposed to a heat flux 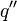 is given by the following equation
is given by the following equation
where k = thermal conductivity (W/(m·K)), ρ = density (kg/m³), and c = specific heat capacity (J/(kg·K)) of the material of interest. 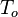 is the temperature, in Kelvin, the material starts at (or the temperature of the bulk material), and
is the temperature, in Kelvin, the material starts at (or the temperature of the bulk material), and 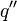 is the heat flux (W/m²) incident to the material.
is the heat flux (W/m²) incident to the material.
Autoignition point of selected substances
Temperatures vary widely in the literature and should only be used as estimates. Factors which may cause variation include partial pressure of oxygen, altitude, humidity, and amount of time required for ignition. Generally the auto-ignition temperature for hydrocarbon/air mixtures increases with increasing molecular weight and increasing chain length. The auto-ignition temperature is also higher for branched-chain hydrocarbons than for straight-chain hydrocarbons.[4]
| Substance | Autoignition (°C)[5] | Autoignition (°F)[5] | Note |
|---|---|---|---|
| Triethylborane | −20 °C | −4 °F | [6] |
| Silane | 21 °C | 70 °F | [6] or below |
| White phosphorus | 34 °C | 93 °F | [6] on contact with an organic substance, melts otherwise |
| Carbon disulfide | 90 °C | 194 °F | [6] |
| Diethyl ether | 160 °C | 320 °F | [7] |
| Gasoline (Petrol) | 247–280 °C | 477–536 °F | [6] |
| Ethanol | 363 °C | 685 °F | [6] |
| Diesel or Jet A-1 | 210 °C | 410 °F | [8] or below |
| Butane | 405 °C | 761 °F | [9] |
| Paper | 218–246 °C | 424–475 °F | [8][10] |
| Leather / Parchment | 200–212 °C | 392–414 °F | [8][11] |
| Magnesium | 473 °C | 883 °F | [6] |
| Hydrogen | 536 °C | 997 °F | [12] |
For paper, there is considerable variation between sources, mainly because there are many physical variables over different kinds of paper, like thickness, density and composition; in addition, it takes longer for the combustion of paper to start at lower temperatures.[13] The novel Fahrenheit 451 is named from a autoignition temperature arbitrarily fixed at this value.[14][15]
See also
- Pyrolysis
- Fire point
- Flash point
- Gas burner (For flame temperatures, combustion heat energy values and ignition temperatures)
References
- ↑ E659 – 78 (Reapproved 2000), "Standard Test Method for Autoignition Temperature of Liquid Chemicals", ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959
- ↑ S. Grynko, "Material Properties Explained" (2012), ISBN 1-4700-7991-7, p. 46.
- ↑ Principles of Fire Behavior. ISBN 0-8273-7732-0. 1998.
- ↑ Zabetakis, M.G. (1965), Flammability characteristics of combustible gases and vapours, U.S. Department of Mines, Bulletin 627.
- 1 2 Under standard conditions for pressure.
- 1 2 3 4 5 6 7 Fuels and Chemicals - Autoignition Temperatures, engineeringtoolbox.com
- ↑ "Diethyl Ether - Safety Properties". Wolfram|Alpha.
- 1 2 3 Cafe, Tony. "PHYSICAL CONSTANTS FOR INVESTIGATORS". tcforensic.com.au. TC Forensic P/L. Retrieved 11 February 2015.
- ↑ "Butane - Safety Properties". Wolfram|Alpha.
- ↑ Tony Cafe. "Physical Constants for Investigators". Journal of Australian Fire Investigators. (Reproduced from "Firepoint" magazine)
- ↑ "Flammability and flame retardancy of leather". leathermag.com. Leather International / Global Trade Media. Retrieved 11 February 2015.
- ↑ "Hydrogen - Safety Properties". Wolfram|Alpha.
- ↑ Forest Products Laboratory (1964). "Ignition and charring temperatures of wood" (PDF). Forest Service U. S. Department of Agriculture.
- ↑ http://www.slate.com/articles/health_and_science/explainer/2012/06/ray_bradbury_death_does_paper_really_burn_at_451_degrees_fahrenheit_.html
- ↑ mastersofmedia.hum.uva.nl/2014/05/19/book-as-a-reflexive-medium-the-materiality-in-post-digital-print/
External links
| ||||||||||||||||||||||||||||||||||
![t_{ig} = \left ( \frac{\pi}{4} \right ) \left (k \rho c \right )\left [ \frac{T_{ig}-T_{o}}{q''} \right]^2](../I/m/1968b7d5a90781ae54f8f0566d0af074.png)