Keutel syndrome
| Keutel syndrome | |
|---|---|
| Classification and external resources | |
| OMIM | 245150 |
| DiseasesDB | 33698 |
Keutel syndrome (KS) is a rare autosomal recessive genetic disorder characterized by abnormal diffuse cartilage calcification, hypoplasia of the mid-face, peripheral pulmonary stenosis, hearing loss, short distal phalanges (tips) of the fingers and mild mental retardation.[1][2][3] Individuals with KS often present with peripheral pulmonary stenosis, brachytelephalangism, sloping forehead, midface hypoplasia, and receding chin. It is associated with abnormalities in the gene coding for matrix gla protein (MGP).[1] Being an autosomal recessive disorder, it may be inherited from two unaffected, abnormal MGP-carrying parents. Thus, people who inherit two affected MGP genes will likely inherit KS.
It was first identified in 1972 as a novel rare genetic disorder sharing similar symptoms with chondrodysplasia punctata.[1][4] Multiple forms of chondrodysplasia punctata share symptoms consistent with KS including abnormal cartilage calcification, forceful respiration, brachytelephalangism, hypotonia, psychomotor delay, and conductive deafness, yet peripheral pulmonary stenosis remains unique to KS.[5]
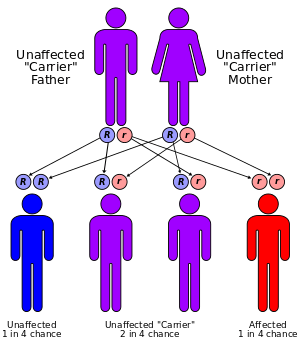
No chromosomal abnormalities are reported in affected individuals, suggesting that familial consanguinity relates to the autosomal recessive mode of inheritance. Also, despite largely abnormal calcification of regions including the larynx, tracheobronchial tree, nose, pinna (anatomy), and epiglottis, patients exhibit normal serum calcium and phosphate levels.[6]
Signs and symptoms
Being an extremely rare autosomal genetic disorder, differential diagnosis has only led to several cases since 1972. Initial diagnosis lends itself to facial abnormalities including sloping forehead, maxillary hypoplasia, nasal bridge depression, wide mouth, dental maloclusion, and receding chin.[7] Electroencephalography (EEG), computed tomography (CT) scanning, and skeletal survey are further required for confident diagnosis. Commonly, diffuse cartilage calcification and brachytelephalangism are identified by X-radiation (X-ray), while peripheral pulmonary arterial stenosis, hearing loss, dysmorphic facies, and mental retardation are confirmed with confidence by the aforementioned diagnostic techniques.[6]
Skeletal effects
Diagnosis is often confirmed by several abnormalities of skeletal origin. There is a sequential order of findings, according to Cormode et al., which initiate in abnormal cartilage calcification and later brachytelephalangism.[8] The uniqueness of brachytelephalangy in KS results in distinctively broadened and shortened first through fourth distal phalanges, while the fifth distal phalanx bone remains unaffected.[9] Radiography also reveals several skeletal anomalies including facial hypoplasia resulting in underdevelopment of the nasal bridge with noticeably diminished alae nasi. In addition to distinguishable facial features, patients generally demonstrate shorter than average stature and general mild developmental delay.
Cartilaginous effects
Many common effects sharing similarity with chondrodysplasia punctata stem from cartilaginous origin. Radiography reveals extensive diffuse cartilaginous calcification. Pulmonary angiography and soft tissue radiography often demonstrate significant cartilaginous ossification in the trachea and larynx, with perichondral and endochondral centers significantly ossified in transformed cartilage.[10] Abnormal diffuse cartilaginous ossification is typically most pronounced in the auricles and cartilage of the trachea and larynx, while peripheral pulmonary stenosis is frequently common in KS. Interestingly, in consanguineous parents of children with KS, one is often phenotypically normal, while the other is positive for pulmonary stenosis. Perhaps emanating from diffuse laryngotracheal calcification, patients often present with recurrent respiratory infection, otitis media, and sinusitis.[11]
Cardiovascular effects
Apart from diffuse abnormal cartilaginous calcification in pulmonary and wikt:otic systems, patients develop significant arterial calcification throughout the body.[12] Such calcification is concomitant with various diseases including diabetes, atherosclerosis, and renal dysfunction, while patients with oral anticoagulant use have significant aortic valve and coronary artery calcification.[12][13] Although not distinctive to KS, echocardiogram analysis has revealed right ventricular hypertrophy resulting in severe pulmonary artery hypertension in several cases.[14]
Pathogenesis
Keutel syndrome is an autosomal recessive disorder caused by a novel loss-of-function mutation in the matrix Gla protein gene (MGP). MGP protein resides in the extracellular matrix and is implicated in inhibiting calcification though the repression of bone morphogenetic protein 2 (BMP2). Mutations resulting in loss of consensus donor splice site at exon 2-intron 2 junctions result in significant diffuse calcification of soft tissue cartilage.[14] Extensive diffuse cartilaginous calcification is present in MGP-knockout mice, manifesting in vascular media replacement with a cartilaginous, chondrocyte-like matrix, and ultimately premature death.[15] Conversely, over expression of extracellular MGP effectively abolishes calcification in chondrocytes, suggesting that MGP may function in inhibiting passive calcification in soft tissues.[16] Recent evidence suggests MGP is a vitamin K dependent protein synthesized by chondrocytes and vascular smooth muscle cells, where it potentiates the inhibition of cartilaginous and arterial calcification. Thus, potential vitamin K deficiency, via nutritional deficiency or coumarin-derivative use, would render MGP uncarboxylated and inactive, thus diminishing biological function.[17][18] Arterial calcification resulting from MGP inactivation results in inimical prognosis, commonly seen in patients with diabetes, atherosclerosis, and renal dysfunction.
Treatment and prognosis
Treatment is symptomatic, often addressing indicators associated with peripheral pulmonary artery stenosis. Laryngotracheal calcification resulting in dyspnea and forceful breathing can be treated with bronchodilators including the short and long-acting β2-agonists, and various anticholinergics. Prognosis is good, yet life expectancy depends on the severity and extent of diffuse pulmonary and arterial calcification.
References
- 1 2 3 Munroe PB, Olgunturk RO, Fryns JP, et al. (1999). "Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome". Nat. Genet. 21 (1): 142–4. doi:10.1038/5102. PMID 9916809.
- ↑ Potparic, Olivera; John Gibson (1995). A Dictionary of Congenital Malformations and Disorders. Informa Health Care. p. 98. ISBN 1-85070-577-1.
- ↑ Teebi AS, Lambert DM, Kaye GM, Al-Fifi S, Tewfik TL, Azouz EM (1998). "Keutel syndrome: further characterization and review". Am J Med Genet 78 (2): 182–7. doi:10.1002/(SICI)1096-8628(19980630)78:2<182::AID-AJMG18>3.0.CO;2-J. PMID 9674914.
- ↑ Keutel J, Jorgensen G, Gabriel P (1972). "A new autosomal recessive syndrome: peripheral pulmonary stenoses, brachytelephalangism, neural hearing loss and abnormal cartilage calcifications-ossification". Birth Defects Orig Art Ser VIII (5): 60–68.
- ↑ Ziereisen E, De Munter C, Perlmutter N (1993). "The Keutel syndrome: report of a case and review of the literature". Pediatric Radiology 23: 314–315. doi:10.1007/bf02010925.
- 1 2 Parmar H, Blaser S, Unger S, Yoo, SJ, Papsin B (2006). "Petrified ears in a patient with Keutel syndrome: temporal bone CT findings". Pediatric Radiology 36: 241–243. doi:10.1007/s00247-005-0036-7.
- ↑ Teebi AS, Lambert DM, Kaye GM, Al-Fifi S, Tewfik TL, Azouz EM (1998). "Keutel Syndrome: Further Characterization and Review". American Journal of Medical Genetics 78: 182–187. doi:10.1002/(sici)1096-8628(19980630)78:2<182::aid-ajmg18>3.3.co;2-k.
- ↑ Cormode EJ, Dawson M, Lowry RB (1986). "Keutel syndrome: clinical report and literature review". American Journal of Medical Genetics 24: 289–294. doi:10.1002/ajmg.1320240209.
- ↑ Miller S (2003). "Brachytelephalangy with sparing of the fifth distal phalanx: a feature highly suggestive of Keutel syndrome". Pediatric Radiology 33: 186–189. doi:10.1007/s00247-002-0846-9.
- ↑ Fryns JP, van Flateren A, Mattelaer P, et al. (1984). "Calcification of cartilages, brachytelephalangy, and peripheral pulmonary stenosis. Confirmation of the Keutel syndrome". European Journal of Pediatrics 142: 201–203. doi:10.1007/bf00442449.
- ↑ Khosroshahi HE, Uluoglu O, Olgunturk R, Basaklar C (1989). "Keutel syndrome: a report of four cases". European Journal of Pediatrics 149: 188–191. doi:10.1007/bf01958278.
- 1 2 Meier M, Weng LP, Alexandrakis E, Ruschoff J, Goeckenjan G (2001). "Tracheobronchial stenosis in Keutel syndrome". European Respiratory Journal 17: 566–569. doi:10.1183/09031936.01.17305660.
- ↑ Schurgers LJ, Aebert H, Vermeer C, Bultmann B, Janzen J (2004). "Oral anticoagulant treatment: friend or foe in cardiovascular disease?". Blood 104: 3231–3232. doi:10.1182/blood-2004-04-1277.
- 1 2 Hur DJ, Raymond GV, Kahler SG, Riegert-Johnson DL, Cohen BA, Boyadjiev SA (2005). "A novel MGP mutation in a consanguineous family: Review of the clinical and molecular characteristics of Keutel syndrome". American Journal of Medical Genetics 135A: 36–40. doi:10.1002/ajmg.a.30680.
- ↑ Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G (1997). "Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein". Nature 386: 78–81. doi:10.1038/386078a0. PMID 9052783.
- ↑ Yagami K, Suh JY, Enomoto-Iwamoto M, Koyama E, Abrams WR, Shapiro IM, Pacifici M, Iwamoto M (1999). "Matrix GLA protein is a developmental regulator of chondroctye mineralization and, when constitutively expressed, blocks endochondral and intramembranous ossification in the limb". Journal of Cell Biology 147: 1097–1108. doi:10.1083/jcb.147.5.1097.
- ↑ Crenenburg ECM, Vermeer C, Koos R, Boumans ML, Hackeng TM, Bouwman FG, Kwaijtaal M, Brandenburg VM, Ketteler M, Schurgers LJ (2008). "The circulating inactive form of matrix gla protein (ucMGP) as a biomarker for cardiovascular calcification". Journal of Vascular Research 45: 427–436.
- ↑ Berkner KL, Runge KW (2004). "The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis". Journal of Thrombosis and Haemostasis 2: 2118–2132. doi:10.1111/j.1538-7836.2004.00968.x. PMID 15613016.
External links
- Keutel syndrome at NIH's Office of Rare Diseases
| ||||||||||||||||||||||||||||||||||||||