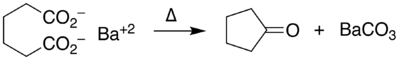Ketonic decarboxylation
Ketonic decarboxylation (also known as ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid to a symmetric ketone by the application of heat with expulsion of one equivalent of water and one equivalent of carbon dioxide. Bases promote this reaction. The reaction mechanism likely involves nucleophilic attack of the alpha-carbon of one acid group on the other acid group's carbonyl, possibly as a concerted reaction with the decarboxylation. The initial formation of an intermediate carbanion with decarboxylation from one acid group prior to the nucleophilic attack has been proposed, but is unlikely since the byproduct resulting from the carbanion's protonation by the acid has never been reported.[1] This reaction is different from oxidative decarboxylation, which proceeds through a radical mechanism and is characterised by a different product distribution in isotopic labeling experiments with two different carboxylic acids. With two different carboxylic acids, the reaction behaves poorly because of poor selectivity except when one of the acids (for example, a small, volatile one) is used in large excess.
Examples
The dry distillation of calcium acetate to acetone was reported by Charles Friedel in 1858 [2] and until World War I ketonization was the premier commercial method for its production.[3]
Ketonic decarboxylation of propanoic acid over a manganese(II) oxide catalyst in a tube furnace.[4] affords the synthesis of 3-pentanone. Of commercial importance is the production of 3-pentanone from propionic acid with catalysts cerium(IV) oxide and manganese dioxide on alumina.
An example of intramolecular ketonization is the conversion of adipic acid to cyclopentanone with barium hydroxide.[5]
References
- ↑ Renz, M (2005). "Ketonization of Carboxylic Acids by Decarboxylation: Mechanism and Scope". Eur. J. Org. Chem. 2005 (6): 979–988. doi:10.1002/ejoc.200400546.
- ↑ Friedel, C. (1858), Ueber s. g. gemischte Acetone. Justus Liebigs Ann. Chem., 108: 122–125. doi:10.1002/jlac.18581080124
- ↑ IMPROVEMENT IN THE MANUFACTURE OF ACETONE.1 E. R. Squibb Journal of the American Chemical Society 1895 17 (3), 187-201 doi:10.1021/ja02158a004
- ↑ Furniss, Brian; Hannaford, Antony; Smith, Peter; and Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed. London: Longman Science & Technical. p. 613. ISBN 9780582462366.
- ↑ J. F. Thorpe and G. A. R. Kon (1925). "Cyclopentanone". Org. Synth. 5: 37.; Coll. Vol. 1, p. 192.