Silyl enol ether

Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group.
Silyl enol ethers are important intermediates in organic synthesis.
Organic synthesis
- Trimethylsilyl enol ethers can be prepared from ketones in presence of a strong base and trimethylsilyl chloride or a weak base and trimethylsilyl triflate.
- Silyl enol ether can form by capturing any enolate formed in a nucleophilic conjugate addition.[1][2]
- A rather exotic way to generate silyl enol ethers is via the Brook rearrangement of appropriate substrates.[3]
Organic reactions
Silyl enol ethers react as nucleophiles in:
- Mukaiyama aldol addition
- Michael reactions
- Alkylations
- Haloketone formation with halogens[4]
- Acyloin formation by organic oxidation with an electrophilic source of oxygen such as an oxaziridine or mCPBA[5]
Saegusa–Ito oxidation
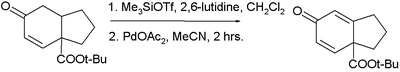
In the Saegusa–Ito oxidation certain silyl enol ethers are oxidized to enones with palladium(II) acetate. In the original publication[6] equal amounts of palladium and 1,4-benzoquinone are used to achieve the reaction with the benzoquinone acting as a co-oxidant. The intermediate is an oxo-allylpalladium complex.
In one application, a dienone is synthesized in two steps from a cyclohexanone:[7][8]
Ring contraction
Cyclic silyl enol ethers have been demonstrated to be viable substrates for regiocontrolled one-carbon ring contractions.[9][10] These reactions employ electron-deficient sulfonyl azides, which undergo chemoselective, uncatalyzed [3+2] cycloaddition to the silyl enol ether, followed by loss of dinitrogen, and alkyl migration to give ring-contracted products in good yield. These reactions may be directed by substrate stereochemistry, giving rise to stereoselective ring-contracted product formation.
Ketene silyl acetals
Ketene silyl acetals are related compounds formally derived from ketenes and acetals with general structure R-C=C(OSiR3)(OR').
References
- ↑ Organic Syntheses, Coll. Vol. 9, p.564 (1998); Vol. 73, p.123 (1996) Article
- ↑ Organic Syntheses, Coll. Vol. 8, p.277 (1993); Vol. 66, p. 43 (1988) Article.
- ↑ Tong, R.; McDonald, F. E. (2008). "Mimicking Biosynthesis: Total Synthesis of the Triterpene Natural Product Abudinol B from a Squalene-like Precursor". Angewandte Chemie (Int. ed.) 47 (23): 4377–4379. doi:10.1002/anie.200800749.
- ↑ Organic Syntheses, Coll. Vol. 8, p.286 (1993); Vol. 69, p.129 (1990) Article
- ↑ Organic Syntheses, Coll. Vol. 7, p.282 (1990); Vol. 64, p.118 (1986) Article.
- ↑ Ito, Yoshihiko; Hirao, Toshikazu & Saegusa, Takeo (1978). "Synthesis of alpha, beta-unsaturated carbonyl compounds by palladium(II)-catalyzed dehydrosilylation of silyl enol ethers". J. Org. Chem. 43 (5): 1011–1013. doi:10.1021/jo00399a052.
- ↑ Clive, Derrick L. J. & Sunasee, Rajesh (2007). "Formation of Benzo-Fused Carbocycles by Formal Radical Cyclization onto an Aromatic Ring". Org. Lett. 9 (14): 2677–2680. doi:10.1021/ol070849l. PMID 17559217.
- ↑ reagents in step 1 are trimethylsilyl triflate and 2,6-lutidine
- ↑ (a) Wohl, R. Helv. Chim. Acta 1973, 56, 1826. (b) Xu, Y. Xu, G.; Zhu, G.; Jia, Y.; Huang, Q. J. Fluorine Chem. 1999, 96, 79.
- ↑ Mitcheltree, M. J.; Konst, Z. A.; Herzon, S. B. Tetrahedron 2013, 69, 5634.