Cycloheximide
 | |
| Names | |
|---|---|
| IUPAC name
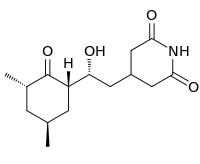
4-[(2R)-2-[(1S,3S,5S)-3,5-Dimethyl-2-oxocyclohexyl]-2-hydroxyethyl]piperidine-2,6-dione | |
| Other names
naramycin a, hizarocin actidione, actispray kaken, U-4527 | |
| Identifiers | |
| 66-81-9 | |
| ChEBI | CHEBI:27641 |
| ChEMBL | ChEMBL123292 |
| ChemSpider | 5962 |
| 5433 | |
| Jmol interactive 3D | Image |
| KEGG | C06685 |
| PubChem | 6197 |
| RTECS number | MA4375000 |
| UNII | 98600C0908 |
| |
| |
| Properties | |
| C15H23NO4 | |
| Molar mass | 281.35 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 119.5 to 121 °C (247.1 to 249.8 °F; 392.6 to 394.1 K) |
| Hazards | |
| Safety data sheet | Oxford MSDS |
| EU classification (DSD) |
Toxic (T) |
| R-phrases | R26 R27 R28 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Cycloheximide is an inhibitor of protein biosynthesis in eukaryotic organisms, produced by the bacterium Streptomyces griseus. Cycloheximide exerts its effect by interfering with the translocation step in protein synthesis (movement of two tRNA molecules and mRNA in relation to the ribosome) thus blocking translational elongation. Cycloheximide is widely used in biomedical research to inhibit protein synthesis in eukaryotic cells studied in vitro (i.e. outside of organisms). It is inexpensive and works rapidly. Its effects are rapidly reversed by simply removing it from the culture medium.
Due to significant toxic side effects, including DNA damage, teratogenesis, and other reproductive effects (including birth defects and toxicity to sperm[1]), cycloheximide is generally used only in in vitro research applications, and is not suitable for human use as a therapeutic compound. Although it has been used as a fungicide in agricultural applications, this application is now decreasing as the health risks have become better understood.
Because cycloheximide is degraded by alkaline conditions (pH > 7), decontamination of work surfaces and containers can be achieved by washing with a non-harmful alkali solution such as soap.
Experimental applications
Cycloheximide can be used as an experimental tool in molecular biology to determine the half-life of a protein. Treating cells with cycloheximide in a time-course experiment followed by Western blotting of the cell lysates for the protein of interest can show differences in protein half-life. Cycloheximide treatment provides the ability to observe the half-life of a protein without confounding contributions from transcription or translation.
It is used as a plant growth regulator to stimulate ethylene production. It is used as a rodenticide and other animal pesticide. It is also used in media to detect unwanted bacteria in beer fermentation by suppressing yeasts and molds growth in test medium.
The translational elongation freezing properties of cycloheximide are also used for ribosome profiling / translational profiling. Translation is halted via the addition of cycloheximide, and the DNA/RNA in the cell is then nuclease treated. The ribosome-bound parts of RNA can then be sequenced.
Spectrum of fungal susceptibility
Cycloheximide has been used to isolate dermatophytes and inhibit the growth of fungi in brewing test media. The following represents susceptibility data for a few commonly targeted fungi:[2]
- Candidia albicans: 12.5 μg/ml
- Mycosphaerella graminicola: 47.2 μg/ml - 85.4 μg/ml
- Saccharomyces cerevisiae: 0.05 μg/ml - 1.6 μg/ml