Johannes Martin Bijvoet
Johannes Martin Bijvoet ForMemRS[1] (23 January 1892, Amsterdam – 4 March 1980, Winterswijk) was a Dutch chemist and crystallographer at the van 't Hoff Laboratory at Utrecht University.[2] He is famous for devising a method of establishing the absolute configuration of molecules.[3][4][5][6][7][8][9] In 1946 he became member of the Royal Netherlands Academy of Arts and Sciences.[10]
The concept of tetrahedrally bound carbon in organic compounds stems back to the work by van 't Hoff and Le Bel in 1874. At this time, it was impossible to assign the absolute configuration of a molecule by means other than referring to the projection formula established by Fischer, who had used glyceraldehyde as the prototype and assigned randomly its absolute configuration.[11]
In 1949 Bijvoet outlined his principle, which relies on the anomalous dispersion of X-ray radiation. Instead of the normally observed elastic scattering of X-rays when they hit an atom, which generates a scattered wave of the same energy but with a shift in phase, X-ray radiation near the absorption edge of an atom creates a partial ionisation process. Some new X-ray radiation is generated from the inner electron shells of the atoms. The X-ray radiation already being scattered is interfered with by the new radiation, both amplitude and phase being altered. These additional contributions to the scattering may be written as a real part 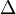 f' and an imaginary one,
f' and an imaginary one, 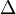 f". Whereas the real part is either positive or negative, the imaginary is always positive, resulting in an addition to the phase angle.
f". Whereas the real part is either positive or negative, the imaginary is always positive, resulting in an addition to the phase angle.
In 1951, using an X-ray tube with a zirconium target, Bijvoet and his coworkers Peerdeman and van Bommel achieved the first experimental determination of the absolute configuration of sodium rubidium tartrate. In this compound, rubidium atoms were the ones close to the absorption edge. In their later publication in Nature, entitled "Determination of the absolute configuration of optically active compounds by means of X-rays", the authors conclude that:
- "The result is that Emil Fisher's convention, which assigned the configuration of FIG. 2 to the dextrorotatory acid appears to answer the reality."
thus confirming the preceding decades of stereochemical assignments. The determination of absolute configuration is nowadays achieved using "soft" X-ray radiation, most often generated with a copper target (which generates X-rays with a characteristic wavelength of 154 pm). Shorter wavelengths make the observable differences in measured intensities smaller, thereby making the distinction of absolute configuration more difficult. The measurement of absolute configuration is also facilitated by the presence of atoms heavier than oxygen.
X-ray diffraction is still considered the ultimate proof of absolute structure, but other techniques such as circular dichroism spectroscopy are often used as faster alternatives.
Bijvoet Center
The Bijvoet Center for Biomolecular Research at Utrecht University, which was founded in 1988, was named after him.[12] The Bijvoet Center performs research on the relation between the structure and function of biomolecules, including proteins and lipids, which play a role in biological processes such as regulation, interaction and recognition.[13] The Bijvoet Center maintains advanced infrastructures for the analysis of proteins using NMR, electron microscopy, X-ray crystallography and mass spectrometry.[14]
References
- ↑ Groenewege, M. P.; Peerdeman, A. F. (1983). "Johannes Martin Bijvoet. 23 January 1892-4 March 1980". Biographical Memoirs of Fellows of the Royal Society 29: 26. doi:10.1098/rsbm.1983.0002. JSTOR 769795.
- ↑ "About Johannes Martin Bijvoet". Bijvoet Center for Biomolecular Research.
- ↑ Bijvoet, J. M.; MacGlllavry, C. H. (1934). "The Crystal Structure of Hg(NH3)2CI2". Nature 134 (3396): 849. doi:10.1038/134849a0.
- ↑ Van Vloten, G. W.; Kruissink, C. . A.; Strijk, B.; Bijvoet, J. M. (1948). "Crystal Structure of 'Gammexane'". Nature 162 (4124): 771. doi:10.1038/162771a0. PMID 18101646.
- ↑ Bijvoet, J. M.; Peerdeman, A. F.; Van Bommel, A. J. (1951). "Determination of the Absolute Configuration of Optically Active Compounds by Means of X-Rays". Nature 168 (4268): 271. doi:10.1038/168271a0.
- ↑ Bijvoet, J. M.; Bernal, J. D.; Patterson, A. L. (1952). "Forty Years of X-Ray Diffraction". Nature 169 (4310): 949. doi:10.1038/169949a0.
- ↑ Bijvoet, J. M. (1954). "Structure of Optically Active Compounds in the Solid State". Nature 173 (4411): 888. doi:10.1038/173888a0.
- ↑ Bijvoet, J. M. Proc. Acad. Sci. Amst. 52, 1949, 313.
- ↑ Peerdeman, A. F., van Bommel, A. J., Bijvoet, J. M. Proc. Acad. Sci. Amst. 54, 1951, 16.
- ↑ "Johannes Martin Bijvoet (1892 - 1980)". Royal Netherlands Academy of Arts and Sciences. Retrieved 26 July 2015.
- ↑ "SODIUM RUBIDIUM (+)-TARTRATE: X-ray crystallography nailed stereochemistry of organic compound". Chemical & Engineering News. 2014.
- ↑ Joop Kessels (April 7, 1988). "SON en RUU in Bijvoet Centrum". Chemische Courant (in Dutch).
- ↑ Erik Hardeman (October 30, 2012). "Bijvoet Centrum (1): ziektes bestrijden op atomair niveau". DUB (in Dutch).
- ↑ "Bijvoet Center - Bijvoet Center for Biomolecular Research". MERIL - Mapping of the European Research Infrastructure Landscape. Retrieved May 2, 2013.
External links
- The Bijvoet Center at Utrecht University
|