Isothiocyanate

Isothiocyanate is the chemical group –N=C=S, formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation.
Synthesis and reactions
The general method for the formation of isothiocyanates proceeds through the reaction between a primary amine (e.g. aniline) and carbon disulfide in aqueous ammonia. This results in precipitation of the ammonium dithiocarbamate salt, which is then treated with lead nitrate to yield the corresponding isothiocyanate.[1] Another method relies on a tosyl chloride mediated decomposition of dithiocarbamate salts that are generated in the first step above.[2]
Isothiocyanates may also be accessed via the thermally-induced fragmentation reactions of 1,4,2-oxathiazoles.[3] This synthetic methodology has been applied to a polymer-supported synthesis of isothiocyanates.[4]
Isothiocyanates are weak electrophiles. Akin to the reactions of carbon dioxide, nucleophiles attack at carbon.
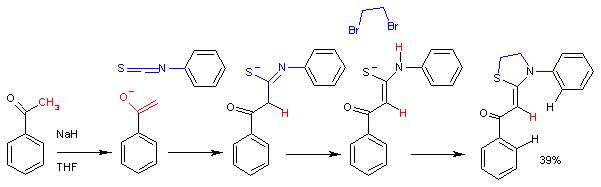
Reflecting their electrophilic character, isothiocyanates are susceptible to hydrolysis.
Biological activity
Isothiocyanates occur widely in nature and are of interest in food science and medicine. Vegetable foods with characteristic flavors due to isothiocyanates include wasabi, horseradish, mustard, radish, Brussels sprouts, watercress, papaya seeds, nasturtiums, and capers. These species generate isothiocyanates in different proportions, and so have different, but recognisably related, flavors. They are all members of the order Brassicales, which is characterised by the production of glucosinolates, and of the enzyme myrosinase, which acts on glucosinolates to release isothiocyanates.
- Sinigrin is the precursor to allyl isothiocyanate
- Glucotropaeolin is the precursor to benzyl isothiocyanate
- Gluconasturtiin is the precursor to phenethyl isothiocyanate
- Glucoraphanin is the precursor to sulforaphane
Phenethyl isothiocyanate (PEITC) and sulforaphane inhibit carcinogenesis and tumorigenesis in certain circumstances. Their mechanism of action is proposed to involve inhibition of cytochrome P450 enzymes, which oxidize compounds such as benzo[a]pyrene and other polycyclic aromatic hydrocarbons (PAHs) into more polar epoxy-diols, which can then cause mutation and induce cancer development.[6] Phenethyl isothiocyanate (PEITC) has been shown to induce apoptosis in certain cancer cell lines, and, in some cases, is even able to induce apoptosis in cells that are resistant to some currently used chemotherapeutic drugs, for example, in drug-resistant leukemia cells that produce the powerful apoptosis inhibitor protein Bcl-2.[7] Furthermore, isothiocyanates have been the basis of a drug in development that replaces the sulfur bonds with selenium, with far stronger potency against melanoma.[8] Certain isothiocyanates have also been shown to bind to the mutated p53 proteins found in many types of tumors, causing an increase in the rate of cell death.[9][10]
The results on the genotoxic effects of the isothiocyanates and glucosinolate precursors are conflicting.[11] Some authors report weak genotoxicity for allyl isothiocyanate and phenethyl isothiocyanate. Induction of point mutations in Salmonella TA98 and TA100, repairable DNA damage in E.coli K-12 cells, and clastogenic effects in mammalian cells by extracts from cruciferous vegetables have also been observed. The goitrogenic effect of Brassicaceae vegetables, interfering with iodine uptake, is also a concern at elevated doses. The average intake of such sulfur-containing compounds through supplementation should not exceed normal levels of consumption.
Coordination chemistry
Isothiocyanate and its linkage isomer thiocyanate are ligands in coordination chemistry. Thiocyanate is more common ligand.

See also
References
- ↑ Dains FB; Brewster RQ; Olander CP (1926). "Phenyl Isothiocyanate". Org. Synth. 6: 72.; Coll. Vol. 1, p. 447
- ↑ Wong, R; Dolman, SJ (2007). "Isothiocyanates from tosyl chloride mediated decomposition of in situ generated dithiocarbamic acid salts". The Journal of Organic Chemistry 72 (10): 3969–3971. doi:10.1021/jo070246n. PMID 17444687.
- ↑ O’Reilly, RJ; Radom, L (2009). "Ab initio investigation of the fragmentation of 5,5-diamino-substituted 1,4,2-oxathiazoles". Organic Letters 11 (6): 1325–1328. doi:10.1021/ol900109b. PMID 19245242.
- ↑ Burkett, BA; Kane-Barber, JM; O’Reilly, RJ; Shi, L (2007). "Polymer-supported thiobenzophenone : a self-indicating traceless 'catch and release' linker for the synthesis of isothiocyanates". Tetrahedron Letters 48 (31): 5355–5358. doi:10.1016/j.tetlet.2007.06.025.
- ↑ Ortega-Alfaro, M. C.; López-Cortés, J. G.; Sánchez, H. R.; Toscano, R. A.; Carrillo, G. P.; Álvarez-Toledano, C. (2005). "Improved approaches in the synthesis of new 2-(1, 3-thiazolidin-2Z-ylidene)acetophenones". Arkivoc 2005 (6): 356–365. doi:10.3998/ark.5550190.0006.631.
- ↑ Zhang, Y; Kensler, TW; Cho, CG; Posner, GH; Talalay, P (1994). "Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates". Proceedings of the National Academy of Sciences of the United States of America 91 (8): 3147–3150. Bibcode:1994PNAS...91.3147Z. doi:10.1073/pnas.91.8.3147. PMC 43532. PMID 8159717.
- ↑ Tsimberidou AM, Keating MJ (Jul 1, 2009). "Treatment of fludarabine-refractory chronic lymphocytic leukemia". Cancer 115 (13): 2824–36. doi:10.1002/cncr.24329. PMID 19402170.
- ↑ Madhunapantula SV, Robertson GP (Mar 23, 2011). "Therapeutic Implications of Targeting AKT Signaling in Melanoma". Enzyme Res 2011: 327923. doi:10.4061/2011/327923. PMC 3065045. PMID 21461351. Lay summary.
- ↑ Wang X, Di Pasqua AJ, Govind S, McCracken E, Hong C, Mi L, Mao Y, Wu JY, Tomita Y, Woodrick JC, Fine RL, Chung FL (Jan 11, 2011). "Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships". J Med Chem 54: 809–816. doi:10.1021/jm101199t. PMID 21241062. (primary source)
- ↑ Wall, Tim (March 10, 2011). "How brocolli fights cancer". Discovery News.
- ↑ Higdon JV, Delage B, Williams DE, Dashwood RH (March 2007). "Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis". Pharmacol Res 55 (3): 224–36. doi:10.1016/j.phrs.2007.01.009. PMC 2737735. PMID 17317210.
- ↑ Gus J. Palenik, George Raymond Clark "Crystal and Molecular Structure of Isothiocyanatothiocyanato-(1-diphenylphosphino-3-dimethylaminopropane)palladium(II)" Inorganic Chemistry, 1970, volume 9, pp 2754–2760. doi:10.1021/ic50094a028
