Iron boride
 | |
| Names | |
|---|---|
| IUPAC name
Iron boride | |
| Other names
Iron monoboride, FeB | |
| Identifiers | |
| 12006-84-7 | |
| Properties | |
| FeB | |
| Molar mass | 66.66 g/mol |
| Appearance | grey powder |
| Density | 7.15 g/cm3 |
| Melting point | 1,300 to 1,500 °C (2,370 to 2,730 °F; 1,570 to 1,770 K) |
| insoluble | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
 | |
| Names | |
|---|---|
| IUPAC name
Iron boride | |
| Other names
Diiron boride, Fe2B | |
| Identifiers | |
| 12006-84-8 | |
| Properties | |
| Fe2B | |
| Molar mass | 122.50 g/mol |
| Appearance | refractory solid |
| insoluble | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron boride refers to various inorganic compounds with the formula FexBy.[1] Two main iron borides are FeB and Fe2B. Some iron borides possess useful properties such as magnetism, electrical conductivity, corrosion resistance and extreme hardness. Some iron borides have found use as hardening coatings for iron. Iron borides have properties of ceramics such as high hardness, and properties of metal properties, such as thermal conductivity and electrical conductivity. Boride coatings on iron are superior mechanical, frictional, and anti-corrosive.[2] Iron monoboride (FeB) is a grey powder that is insoluble in water. FeB is harder than Fe2B, but is more brittle and more easily fractured upon impact.
Formation
Thermochemical formation
Iron borides can be formed by thermochemically reacting boron rich compounds on an iron surface to form a mixture of iron borides, in a process known as boriding. There are a number of ways of forming boride coatings, including gas boriding, molten salt boriding, and pack boriding.[3] Typically carbon tetraboride (B4C) or crystalline boron, is sintered on the iron surface in a tetrafluoroborate flux to form the coatings. The boron atoms diffuse into the iron substrate between 1023 and 1373 K. They first form layers of Fe2B and then form layers of FeB.The range of compounds and compositions formed depends on the reaction conditions including temperature and surrounding environment.[3]
Synthesis
Iron Boride nanoparticles have been formed by reducing iron bromide salts in highly coordinating solvents using sodium borohydride. They have also been prepared by reducing iron salts using sodium borohydride:[4]
- 4FeSO4 + 8NaBH4 +18H2O → 2Fe2B + 6B(OH)3 + 25H2 + 4Na2SO4
Structure
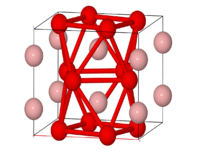
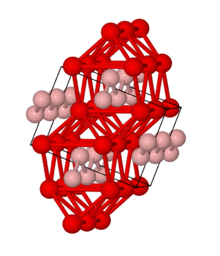
The structures of FeB and Fe2B were known to be interstitial in early studies. FeB has orthorhombic crystal structure.Fe2B adopts body central tetragonal structure.[5]
FeB
FeB has zia-zag chains of boron atoms that are coordinated by seven iron atoms. Boron atoms have a slightly distorted mono-capped trigonal prismatic iron atom coordination and two boron atom neighbors. B-B single bond distance is 178 pm, Fe-B distance is 215-220 pm, and Fe-Fe distance is 240-272 pm. Each trigonal prism shares two rectangular faces with the nearby prisms, forming infinite prism columns. The lattice parameters of boride are a = 0.4061 nm, b = 0.5506 nm, and c = 0.2952 nm.[6]
FeB single crystal is taken by bond domains. Bond domains are parallel to AEM(the axis of easy magnetization) and perpendicular to AHM(the axis of hard magnetization). Structure of closing domains are described as “rows and zigzags of asterisk.” Its bond domains possess a distinguished direction in orientation of the boundaries of major domains with rhombic shape of closing domains.[6]
Fe2B
Fe2B contains single boron atoms in square anti-prismatic iron atom coordination. Boron atoms are separated from each other and the shortest B-B distance is 213 pm. Fe-B distance is 218 pm and Fe-Fe distance is 240-272 pm.[7]
Iron monoboride is only made in single layer. Iron boride (Fe2B) is a submicron and nanopowder that is also insoluble in water. They have been found to be superconductive and ultra-incompressible. It can be made in both single or double layer.[8]
Applications
Boriding is often used to improve abrasion resistance, corrosion resistance, wear resistance, and oxidation resistance. It is used in oil and gas refinery, chemical extraction, automotive, agricultural, stamping, textile extrusion and injection molding industries.[2]
Iron based coatings recently gained attention for their mechanical, frictional, and corrosion resistant properties. As compared to the ceramic or cermet type of materials people have used before, iron based materials are relatively inexpensive, less strategic, and can be produced economically by various thermal methods with ease of fabrication and machining.[9]
See also
References
- ↑ Haynes, William M. Handbook of Chemistry and Physics (91 edition.). 2010, Boca Raton, Florida: CRC Press. ISBN 978-1439820773
- 1 2 "Boriding / Boronizing (DHB)". IBC Coating Technologies. IBC Coating Technologies. Retrieved 17 November 2014.
- 1 2 Keddam, M; Chentouf, SM (2005). "A diffusion model for describing the bilayer growth (FeB/Fe2B) during the iron powder-pack boriding". Appl. Surf. Sci. 252: 393–399. doi:10.1016/j.apsusc.2005.01.016.
- ↑ Alyoshin, V.G. (1981). Investigation of Composition and Chemical State of Elements in Iron Boride by the Method of X-Ray Photoelectron Spectroscopy. 38. Journal of Solid State Chemistry. pp. 105, 111. doi:10.1016/0022-4596(81)90478-3.
- ↑ Joshi, A.A.;Hosmani, S.S. (2014). Pack-Boronizing of AISI 4140 Steel: Boronizing Mechanism and the Role of Container Design. Materials and Manufacturing Processes Volume 29, Issue 9. doi:10.1080/10426914.2014.921705.
- 1 2 Lyakhova, M.B.; Pastushenkov, Y. G.; Zhdanova, O.V. (May 2013). Magnetic Properties and Domain Structure of FeB Single Crystals. Metal Science and Heat Treatment, Vol. 55, Nos. 1 – 2. doi:10.1007/s11041-013-9581-0.
- ↑ Kapfenberger, C.; Albert, B.; Pottgen, R.; Huppertz, H. (2006). Structure refinements of iron borides Fe2B and FeB.ls. Z. Kristallogr. 221. doi:10.1524/zkri.2006.221.5-7.477.
- ↑ Iron Boride; MSDS No.12006-84-7;ESPI Metals: Ashland, OR, Mar, 1990; http://www.espimetals.com/index.php/msds/592-iron-boride
- ↑ Zhdanova, O.V.; Lyakhova, M.B.; Pastushenkov, Y.G. (May 2013). "Magnetic Properties and Domain Structure of FeB Single Crystals". Met. Sci. Heat Treat. 55: 1–2. doi:10.1007/s11041-013-9581-0.