Ion chromatography
| Acronym | IC, IEC |
|---|---|
| Classification | Chromatography |
| Other techniques | |
| Related |
High performance liquid chromatography Aqueous Normal Phase Chromatography Size exclusion chromatography Micellar liquid chromatography |
Ion-exchange chromatography (or ion chromatography) is a chromatography process that separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. It is often used in protein purification, water analysis, and quality control.
History

The boom of Ion exchange chromatography primarily began between 1935-1950 and it was through the "Manhattan project" that applications and IC were significantly extended. It was in the fifties and sixties that theoretical models were developed for IC for further understanding and it was not until the seventies that continuous detectors were utilized, paving the path for the development from low-pressure to high-performance chromatography. Not until 1975 was "ion chromatography" was established as a name in reference to the techniques, and was thereafter used as a name for marketing purposes. Today IC is important for investigating aqueous systems, such as drinking water. It is a popular method for analyzing anionic elements or complexes that help solve environmentally relevant problems. Likewise, it also has great uses in the semiconductor industry.
Because of the abundant separating columns, elution systems, and detectors available, chromatography has developed into the main method for ion analysis.[1]
Principle
Ion-exchange chromatography retains analyte molecules on the column based on coulombic (ionic) interactions. The stationary phase surface displays ionic functional groups (R-X) that interact with analyte ions of opposite charge. This type of chromatography is further subdivided into cation exchange chromatography and anion-exchange chromatography. The ionic compound consisting of the cationic species M+ and the anionic species B- can be retained by the stationary phase.
Cation exchange chromatography retains positively charged cations because the stationary phase displays a negatively charged functional group:
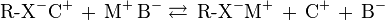
Anion exchange chromatography retains anions using positively charged functional group:
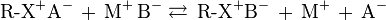
Note that the ion strength of either C+ or A- in the mobile phase can be adjusted to shift the equilibrium position, thus retention time.
The ion chromatogram shows a typical chromatogram obtained with an anion exchange column.
Typical technique

A sample is introduced, either manually or with an autosampler, into a sample loop of known volume. A buffered aqueous solution known as the mobile phase carries the sample from the loop onto a column that contains some form of stationary phase material. This is typically a resin or gel matrix consisting of agarose or cellulose beads with covalently bonded charged functional groups. The target analytes (anions or cations) are retained on the stationary phase but can be eluted by increasing the concentration of a similarly charged species that displaces the analyte ions from the stationary phase. For example, in cation exchange chromatography, the positively charged analyte can be displaced by adding positively charged sodium ions. The analytes of interest must then be detected by some means, typically by conductivity or UV/Visible light absorbance.
Control an IC system usually requires a chromatography data system (CDS). In addition to IC systems, some of these CDSs can also control gas chromatography (GC) and HPLC
Separating proteins

Proteins have numerous functional groups that can have both positive and negative charges. Ion exchange chromatography separates proteins by their net charge, which depends on the composition of the mobile phase. By adjusting the pH or the ionic concentration of the mobile phase, various protein molecules can be separated. For example, if a protein has a net positive charge at pH 7, it binds to a column of negatively charged beads—whereas a negatively charged protein does not. By changing the pH so that the net charge on the protein is negative, it too is eluted.
Elution by increasing ionic strength of the mobile phase is more subtle. It works because ions from the mobile phase interact with the immobilized ions on the stationary phase, thus "shielding" the stationary phase from the protein, and letting the protein elute.
Separation can be achieved based on the natural isoelectric point of the protein. Alternatively a peptide tag can be genetically added to the protein to give the protein an isoelectric point away from most natural proteins (e.g., 6 arginines for binding to a cation-exchange resin or 6 glutamates for binding to an anion-exchange resin such as DEAE-Sepharose).
Elution from ion-exchange columns can be sensitive to changes of a single charge- chromatofocusing. Ion-exchange chromatography is also useful in the isolation of specific multimeric protein assemblies, allowing purification of specific complexes according to both the number and the position of charged peptide tags.[2][3]
Uses
Clinical utility
Used in measurement of HbA1c, porphyrin & water purification.
Industrial Applications
Allows for quantitative testing of electrolyte and proprietary additives of electroplating baths.[4] It is an advancement of qualitative hull cell testing or less accurate UV testing. Ions, catalysts, brighteners and accelerators can be measured.[4]
See also
- Isoelectric point
- High performance liquid chromatography
- Chromatofocusing
- Anion-exchange chromatography
References
- ↑ Eith, Claudia, Kolb Maximilian, and Seubert Andreas. "Introduction." Practical Ion Chromatography An Introduction. Ed. Viehweger Kai. Herisau: Metrohm, 2002. 160.
- ↑ Sakash, J.B.; Kantrowitz, E.R. (2000). "The contribution of individual interchain interactions to the stabilization of the T and R states of Escherichia coli aspartate transcarbamoylase.". J Biol Chem 275 (37): 28701–7. doi:10.1074/jbc.M005079200. PMID 10875936.
- ↑ Fairhead, M. (2013). "Plug-and-Play Pairing via Defined Divalent Streptavidins.". J Mol Biol 426 (1): 199–214. doi:10.1016/j.jmb.2013.09.016. PMID 24056174.
- 1 2 Robert E. Smith (31 December 1987). Ion Chromatography Applications. CRC Press. ISBN 978-0-8493-4967-6.
Bibliography
- Small, Hamish (1989). Ion chromatography. New York: Plenum Press. ISBN 0-306-43290-0.
- Tatjana Weiss; Weiss, Joachim (2005). Handbook of Ion Chromatography. Weinheim: Wiley-VCH. ISBN 3-527-28701-9.
- Gjerde, Douglas T.; Fritz, James S. (2000). Ion Chromatography. Weinheim: Wiley-VCH. ISBN 3-527-29914-9.
- Jackson, Peter; Haddad, Paul R. (1990). Ion chromatography: principles and applications. Amsterdam: Elsevier. ISBN 0-444-88232-4.
External links
| Library resources about Ion exchange chromatography |
| ||||||||||||||||||||||
|