Investigational New Drug
| Regulation of therapeutic goods in the United States |
|---|
 |
|
Prescription drugs Over-the-counter drugs |
|
Non-governmental organizations |
The United States Food and Drug Administration's Investigational New Drug (IND) program is the means by which a pharmaceutical company obtains permission to ship an experimental drug across state lines (usually to clinical investigators) before a marketing application for the drug has been approved. The FDA reviews the IND application for safety to assure that research subjects will not be subjected to unreasonable risk. If the application is cleared, the candidate drug usually enters a Phase 1 clinical trial. Regulations are primarily at 21 C.F.R. 312.
Criteria for application
An IND is required for a clinical study if it is intended to support a:
- New indication
- Change in the approved route of administration or dosage level
- Change in the approved patient population (e.g. pediatric) or a population at greater or increase of risk (elderly, HIV positive, immunocompromised)
- Significant change in the promotion of an approved drug
Application contents
The IND application must contain information in three broad areas:[1]
- Animal Pharmacology and Toxicology Studies – Preclinical data to permit an assessment as to whether the product is reasonably safe for initial testing in humans. Also included are any previous experience with the drug in humans (often foreign use).
- Chemistry and Manufacturing Information – Information pertaining to the chemical composition, manufacturing methods, stability, and controls used for manufacturing the drug substance and the drug product. The chemical stability and activity of the product must also have been tested. This information is assessed to ensure that the company can adequately produce and supply consistent and active batches of the drug.
- Clinical Protocols and Investigator Information – Detailed protocols for proposed clinical studies to assess whether the initial-phase trials will expose the subjects to unnecessary risks. Information on the qualifications of clinical investigators—professionals (generally physicians) who oversee the administration of the experimental compound—to assess whether they are qualified to fulfill their clinical trial duties. Finally, commitments to obtain informed consent from the research subjects, to obtain review of the study by an institutional review board (IRB), and to adhere to the investigational new drug regulations.
An IND must also include an Investigator's Brochure which is a document intended to educate the trial investigators of the significant facts about the trial drug they need to know to conduct their clinical trial with the least hazard to the subjects or patients who will be enrolled.
IND types
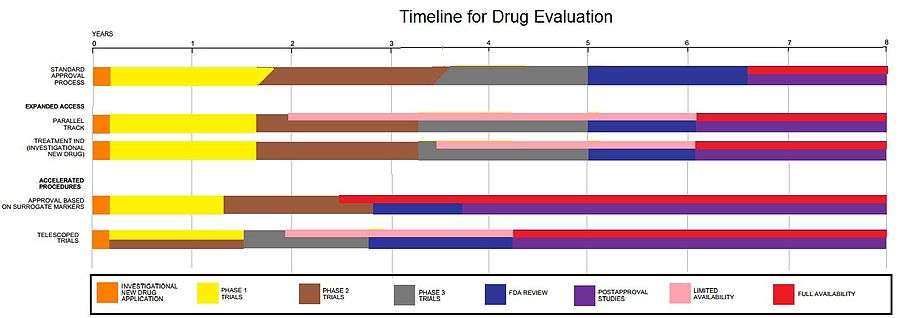
There are three IND types:[1]
- An Investigator IND is submitted by a physician who both initiates and conducts an investigation, and under whose immediate direction the investigational drug is administered or dispensed. A physician might submit a research IND to propose studying an unapproved drug, or an approved product for a new indication or in a new patient population.
- Emergency Use IND allows the FDA to authorize use of an experimental drug in an emergency situation that does not allow time for submission of an IND.
- Treatment IND is submitted for experimental drugs showing promise in clinical testing for serious or immediately life-threatening conditions while the final clinical work is conducted and the FDA review takes place.
Additional regulations
- Experimental drugs under an IND must be labeled, "Caution: New Drug – Limited by Federal (or United States) law to investigational use.
Noteworthy examples
The FDA runs a medical marijuana IND program (the Compassionate Investigational New Drug program). It stopped accepting new patients in 1992 after public health authorities concluded there was no scientific value to it, and due to President George H.W. Bush administration's desire to "get tough on crime and drugs." As of 2011, four patients continue to receive cannabis from the government under the program.[2]
See also
- Abigail Alliance for Better Access to Developmental Drugs
- Drug Discovery
- Expanded access ("compassionate use")
- FDA Fast Track Development Program
- Good Manufacturing Practice
- Inverse benefit law
- New drug application, after clinical trials
- Executive Order 13139, regulates the application of IND drugs to US service personnel.
References
- 1 2 "Investigational New Drug (IND) Application". FDA. Retrieved 7 March 2013.
- ↑ AP (September 27, 2011). Associated Press News http://www.cbsnews.com/news/4-americans-get-medical-pot-from-the-feds/. Missing or empty
|title=(help)
- Investigational New Drug (IND) Application Process Center for Drug Evaluation and Research, Food and Drug Administration.
- ICH Guidance for Industry, E6 Good Clinical Practice: Consolidated Guidance. BROKEN LINK
- Troetel, W.M.: Achieving a Successful US IND Filing (1) The Regulatory Affairs Journal. 6: 22–28, January 1995.
- Troetel, W.M.: Achieving a Successful US IND Filing (2) The Regulatory Affairs Journal. 6: 104–108, February 1995.
Further reading
- Henninger, Daniel (2002). "Drug Lag". In David R. Henderson (ed.). Concise Encyclopedia of Economics (1st ed.). Library of Economics and Liberty. OCLC 317650570, 50016270 and 163149563