Guanfacine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
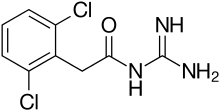
N-(diaminomethylidene)-2-(2,6-dichlorophenyl)acetamide | |
| Clinical data | |
| Trade names | Afken, Estulic, Intuniv, Tenex |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a601059 |
| Licence data | US Daily Med:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | oral, intravenous |
| Pharmacokinetic data | |
| Bioavailability | 80-100% (IR), 58% (XR)[1][2] |
| Protein binding | 70%[1][2] |
| Metabolism | CYP3A4[1][2] |
| Biological half-life | IR: 10-17 hours; XR: 17 hours (10-30) in adults & adolescents and 14 hours in Paediatrics[1][2][3][4] |
| Excretion | renal (80%; 50% [range: 40-75%] as unchanged drug)[1][2] |
| Identifiers | |
| CAS Number |
29110-47-2 |
| ATC code | C02AC02 |
| PubChem | CID 3519 |
| IUPHAR/BPS | 522 |
| DrugBank |
DB01018 |
| ChemSpider |
3399 |
| UNII |
30OMY4G3MK |
| KEGG |
D08031 |
| ChEMBL |
CHEMBL862 |
| Chemical data | |
| Formula | C9H9Cl2N3O |
| Molar mass | 246.093 g/mol |
| |
| |
| | |
Guanfacine (brand name Estulic, Tenex and the extended release Intuniv) is a sympatholytic drug used in the treatment of attention deficit hyperactivity disorder (ADHD) and hypertension (not to be confused with guaifenesin, an expectorant).[5] It is a selective α2A receptor agonist. These receptors are concentrated heavily in the prefrontal cortex and the locus coeruleus, with the potential to improve attention resulting from interaction with receptors in the former.[6] Guanfacine lowers both systolic and diastolic blood pressure by activating the central nervous system α2A norepinephrine autoreceptors, which results in reduced peripheral sympathetic outflow and thus a reduction in peripheral sympathetic tone.[7]
Guanfacine is currently approved and marketed in the United States[8] and Europe[9] as Intuniv for the treatment of ADHD in children and adolescents aged 6–18 years.
Medical uses
Hypertension
It has been shown to reduce hypertension not just in the short-term, but also in long-term studies to be able to achieve normalization in the blood pressure of 54% of patients treated over a year and 66% over two years. The average reduction in mean arterial pressure of all patients was 16% at the end of the first year and 17% at the end of the second year. [10]

Attention deficit hyperactivity disorder
The US Food and Drug Administration (FDA) has approved guanfacine for the treatment of attention deficit hyperactivity disorder (ADHD), like clonidine, alone or with stimulants in 2010, for pediatric patients aged 6–17 years.[11] Its beneficial actions are likely due to its ability to strengthen prefrontal cortical regulation of attention and behavior.[12] Guanfacine is also used in conjunction with stimulants to augment therapeutic actions, reduce rebound, and when taken at evening, to induce sleep.[13] Guanfacine is thought to improve regulation of behavior, attention, and emotion through actions at post-synaptic α2A adrenergic receptors on prefrontal cortical neurons, which strengthen prefrontal cortical network connections.[14] In animal models, guanfacine is seen to affect a number of cognitive factors, including working memory improvement, distractibility reduction, response inhibition improvement, and enhanced attention and impulse control. Improvement is most evident in subjects with cognitive deficits; more subtle effects are seen in healthy young animals.[12] Performance increases in spatial working memory have also been observed in humans.[15] Another study found no effect on healthy male adults' executive functions and working memory, and small decrements on 2 tasks relating to the sedative effect of guanfacine.[16] Recent studies suggest that guanfacine may help patients with a variety of prefrontal cortical disorders, including traumatic brain injury to the frontal lobe,[17] strokes affecting higher cognitive function,[18] and dysregulated emotional behavior, e.g. in children who have been traumatized.[19]
Side effects of guanfacine are dose-dependent.[20]
Anxiety
Another psychiatric use of guanfacine is for treatment of anxiety, such as generalized anxiety disorder and post-traumatic stress disorder symptoms. Guanfacine and other α2A agonists have anxiolytic-like action,[21] reducing sympathetic arousal in the emotional responses of the amygdala, and strengthen prefrontal cortical regulation of emotion, action and thought. Due to its prolonged half-life, it also has been seen to improve sleep interrupted by nightmares in PTSD patients.[22] All of these actions likely contribute to the relief of the hyperarousal, re-experiencing of memory, and impulsivity associated with PTSD.[23] However, a recent study showed no benefit for patients suffering from PTSD.[24]
Tics
According to recent studies (Srour et al., 2008) there is controversy as to guanfacine’s usefulness in treating tics. There has been success when tic symptoms are co-morbid with ADHD, and as such, guanfacine and other α2A-adrenergic agonists (clonidine) are commonly the first choice for treatment.
Treatment of withdrawal syndrome
Guanfacine is also being investigated for treatment of withdrawal for opioids, ethanol, and nicotine.[25]
Adverse effects
Adverse effects incidence:[2]
Very common (>10% incidence) adverse effects include:
- Xerostomia (dry mouth)
- Somnolence (sleepiness)
- Fatigue
- Dizziness
- Headache
- Constipation
- Abdominal pain
Common (1-10% incidence) adverse effects include:
- Alopecia (hair loss)
- Asthenia (weakness)
- Bradycardia (low heart rate)
- Confusion
- Increased appetite
- Depression
- Dermatitis
- Diaphoresis (sweating without a physiologic reason)
- Dizziness
- Dyspepsia (indigestion)
- Dysphagia (being unable to swallow)
- Dyspnoea (air hunger)
- Hypokinesia
- Hypotension (low blood pressure)
- Impotence
- Insomnia
- Irritability
- Leg cramps
- Lethargy
- Nausea
- Palpitations
- Pruritus (itching)
- Tachycardia (high heart rate)
- Weakness
Unknown frequency adverse effects include:
- Abnormal liver function tests
- Agitation
- Alterations in taste
- Anxiety
- Arthralgia (joint pain)
- Blurred vision
- Chest pain
- Confusion
- Diarrhoea
- Exfoliation
- Exfoliative dermatitis
- Hallucinations
- Impotence
- Leg pains
- Malaise
- Myalgia (muscle pain)
- Nervousness
- Nocturia (waking to urinate)
- Oedema (swelling)
- Orthostatic hypotension
- Paresthesia (pins and needles)
- Rash
- Tremor
- Urinary frequency
- Vertigo (dizziness)
Interactions
- CYP3A4/5 inhibitors — Use caution. Elevates plasma concentration of guanfacine.
- CYP3A4 inducers — Recommended increase in guanfacine dose.
- Valproic acid — Use caution. Elevates plasma concentration of valproic acid.
- Antihypertensive drugs — Use caution. Potential for additive pharmacodynamic effects (hypotension, syncope, etc.)
- CNS depressant drugs — Use caution. Potential for additive pharmacodynamic effects (sedation, somnolence, etc.)
Pharmacokinetics and metabolism
Guanfacine has an oral bioavailability of 80%. There is no clear evidence of any first-pass metabolism. Elimination half-life is 17 hours with the major elimination route being renal. The principal metabolite is the 3-hydroxy-derivative, with evidence of moderate biotransformation, and the key intermediate being an epoxide.[26] It is also shown that elimination in patients with impaired renal function does not differ significantly from those with normal renal function. As such, metabolism by liver is the assumption for those with impaired renal function, as supported by increased frequency of known side effects of orthostatic hypotension and sedation.[27] In animal models, guanfacine’s enhancing effects on the working-memory functions of the pre-frontal cortex are thought to be due to inhibition of cAMP-mediated signaling, which is effected by the Gi proteins that are generally coupled to the post-synaptic alpha-2a-adrenoceptors that guanfacine stimulates through binding.[28]
Pharmacology
Guanfacine is a highly selective agonist of the α2A adrenergic receptor, with negligible affinity for any other receptor.[29] However, it may also be a potent 5-HT2B receptor agonist, potentially contributing to valvulopathy.[30]
| Receptor | Ki (nM) |
|---|---|
| α2A | 71.8 |
| α2B | 1200.2 |
| α2C | 2505.2 |
See also
Notes and references
- 1 2 3 4 5 "Guanfacine (guanfacine) Tablet [Genpharm Inc.]". DailyMed. Genpharm Inc. March 2007. Retrieved 9 November 2013.
- 1 2 3 4 5 6 "guanfacine (Rx) - Intuniv, Tenex". Medscape Reference. WebMD. Retrieved 9 November 2013.
- ↑ Hofer, Kristi N.; Buck, Marcia L. (2008). "New Treatment Options for Attention-Deficit/Hyperactivity Disorder (ADHD): Part II. Guanfacine". Pediatric Pharmacotherapy (Medscape) (14): 4.
- ↑ Cruz, MP (Aug 2010). "Guanfacine Extended-Release Tablets (Intuniv), a Nonstimulant Selective Alpha(2A)-Adrenergic Receptor Agonist For Attention-Deficit/Hyperactivity Disorder.". P & T : a peer-reviewed journal for formulary management 35 (8): 448–51. PMC 2935643. PMID 20844694.
- ↑ monograph
- ↑ Kolar, Dusan; Keller, Amanda; Golfinopoulos, Maria; Cumyn, Lucy; Syer, Cassidy & Hechtman, Lily (2008), "Treatment of adults with attention-deficit/hyperactivity disorder", Neuropsychiatric Disease and Treatment 4 (2): 389–403, PMC 2518387, PMID 18728745.
- ↑ Van Zwieten, P.; Thoolen, M. & Timmermans, P. (1983), "The pharmacology of centrally acting antihypertensive drugs", British Journal of Clinical Pharmacology 15 (Suppl 4): 455S–462S, doi:10.1111/j.1365-2125.1983.tb00311.x, PMC 1427667.
- ↑ "FDA Approves Intuniv". Drugs.com. September 2009.
- ↑ "European Medicines Agency: Intuniv". ema.europa.eu. October 2015.
- ↑ Jerie, P. (1980). "Clinical experience with guanfacine in long-term treatment of hypertension: Part I: efficacy and dosage". British Journal of Clinical Pharmacology 10 (Suppl 1): 37S–47S. doi:10.1111/j.1365-2125.1980.tb04903.x. PMC 1430120. PMID 6994777..
- ↑ Kornfield R, Watson S, Higashi A, Dusetzina S, Conti R, Garfield R, Dorsey ER, Huskamp HA, Alexander GC; Watson; Higashi; Conti; Dusetzina; Garfield; Dorsey; Huskamp; Alexander (April 2013). "Impact of FDA Advisories on Pharmacologic Treatment of Attention Deficit Hyperactivity Disorder". Psychiatric Services 64 (4): 339–46. doi:10.1176/appi.ps.201200147. PMC 4023684. PMID 23318985.
- 1 2 Arnsten AF (October 2010), "The use of α2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder", Expert Rev Neurother 10 (10): 1595–605, doi:10.1586/ern.10.133, PMC 3143019, PMID 20925474
- ↑ Zito, Julie M.; Derivan, Albert T.; Kratochvil, Christopher J.; Safer, DJ; et al. (2008), "Off-label psychopharmacologic prescribing for children: History supports close clinical monitoring", Child and Adolescent Psychiatry and Mental Health 2 (1): 24, doi:10.1186/1753-2000-2-24, PMC 2566553, PMID 18793403
- ↑ Wang, M; Ramos, BP (2007), "Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex", Cell 129 (2): 397–410, doi:10.1016/j.cell.2007.03.015, PMID 17448997
- ↑ Jäkälä P; Riekkinen M; Sirviö J (May 1999), "Guanfacine, but not clonidine, improves planning and working memory performance in humans", Neuropsychopharmacology 20 (5): 460–70, doi:10.1016/S0893-133X(98)00127-4, PMID 10192826
- ↑ Müller, U; Clark, L; Lam, ML; Moore, RM; Murphy, CL; Richmond, NK; Sandhu, RS; Wilkins, IA; Menon, DK; et al. (2005), "Lack of effects of guanfacine on executive and memory functions in healthy male volunteers", Psychopharmacology 182 (2): 205–13, doi:10.1007/s00213-005-0078-4, PMID 16078088.
- ↑ McAllister TW; McDonald BC; Flashman LA (October 2011), "Alpha-2 adrenergic challenge with guanfacine one month after mild traumatic brain injury: altered working memory and BOLD response", Int J Psychophysiol 82 (1): 107–14, doi:10.1016/j.ijpsycho.2011.06.022, PMC 3210922, PMID 21767584
- ↑ Singh-Curry V, Malhotra P, Farmer SF, Husain M; Malhotra; Farmer; Husain (June 2011), "Attention deficits following ADEM ameliorated by guanfacine", J. Neurol. Neurosurg. Psychiatr. 82 (6): 688–90, doi:10.1136/jnnp.2009.195792, PMC 3090126, PMID 20562462
- ↑ Connor DF; Findling RL; Kollins SH (September 2010), "Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial", CNS Drugs 24 (9): 755–68, doi:10.2165/11537790-000000000-00000, PMID 20806988
- ↑ Jerie, P. (1980), "Clinical experience with guanfacine in long-term treatment of hypertension: Part II: adverse reactions to guanfacine", British Journal of Clinical Pharmacology 10 (Suppl 1): 157S–164S, doi:10.1111/j.1365-2125.1980.tb04924.x, PMC 1430125, PMID 6994770.
- ↑ Brain Res. 2004 Nov 19;1027(1-2):173-8. Noradrenergic alpha-2 agonists have anxiolytic-like actions on stress-related behavior and mesoprefrontal dopamine biochemistry. Morrow BA, George TP, Roth RH
- ↑ Kozarlc-Kovaclc, D. (2008), "Psychopharmacotherapy of Posttraumatic Stress Disorder", Croatian Medical Journal 49 (4): 459–475, doi:10.3325/cmj.2008.4.459, PMC 2525822, PMID 18716993.
- ↑ Kaminer, D.; Seedat, S. & Stein, D. (2005), "Post-traumatic stress disorder in children", World Psychiatry 4 (2): 121–5, PMC 1414752, PMID 16633528.
- ↑ Neylan,, T.; Lenoci, M. & Samuelson, K. (2006), "No Improvement of Posttraumatic Stress Disorder Symptoms With Guanfacine Treatment", American Journal of Psychiatry 163 (12): 2186–2188, doi:10.1176/appi.ajp.163.12.2186, PMID 17151174
- ↑ Sofuogul, M. & Sewell, A. (2009), "Norepinephrine and Stimulant Addiction", Addiction Biology 14 (2): 119–129, doi:10.1111/j.1369-1600.2008.00138.x, PMC 2657197, PMID 18811678
- ↑ Kiechel, J. (1980), "Pharmacokinetics and metabolism of guanfacine in man: A review", British Journal of Clinical Pharmacology 10 (Suppl 1): 25S–32S, doi:10.1111/j.1365-2125.1980.tb04901.x, PMC 1430131, PMID 6994775.
- ↑ Kirch, W.; Kohler, H. & Braun, W. (1980), "Elimination of guanfacine in patients with normal and impaired renal function", British Journal of Clinical Pharmacology 10 (Suppl 1): 33S–35S, doi:10.1111/j.1365-2125.1980.tb04902.x, PMC 1430110, PMID 6994776.
- ↑ Ramos, Brian P.; Stark, David; Verduzco, Luis; van Dyck, Christopher H.; Arnsten, Amy F. T. (2006), "α2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals", Learning & Memory 13 (6): 770–6, doi:10.1101/lm.298006, PMC 1783631, PMID 17101879.
- ↑ Roth, BL; Driscol, J (12 January 2011), "PDSP Ki Database", Psychoactive Drug Screening Program (PDSP) (University of North Carolina at Chapel Hill and the United States National Institute of Mental Health), retrieved 15 November 2013
- ↑ Xi-Ping Huang;
Vincent Setola;
Prem N. Yadav;
John A. Allen;
Sarah C. Rogan;
Bonnie J. Hanson;
Chetana Revankar;
Matt Robers;
Chris Doucette;
Bryan L. Roth (29 June 2009), Parallel Functional Activity Profiling Reveals Valvulopathogens Are Potent 5-Hydroxytryptamine2B Receptor Agonists: Implications for Drug Safety Assessment, ASPET Journals, retrieved 1 January 2014 line feed character in
|author=at position 15 (help)
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||