Intracytoplasmic sperm injection
| Intracytoplasmic sperm injection | |
|---|---|
| Intervention | |
|
Oocyte is injected during ICSI | |
| MeSH | D020554 |
Intracytoplasmic sperm injection (ICSI, pronounced /ɪksiː/, IK-see) is an in vitro fertilization procedure in which a single sperm is injected directly into an egg. Defective sperm function remains the single most important cause of human infertility ([1]). Although certain severe forms of male infertility have a genetic origin, others may be the result of environmental factors. During the past decade, ICSI has been applied increasingly around the world to alleviate problems of severe male infertility in human patients who either could not be assisted by conventional IVF procedures or could not be accepted for IVF because too few motile and morphologically normal sperm were present in the ejaculate of the male partner ([2]).
Indications
This procedure is most commonly used to overcome male infertility problems, although it may also be used where eggs cannot easily be penetrated by sperm, and occasionally in addition to sperm donation.[3]
It can be used in teratozoospermia, because once the egg is fertilized, abnormal sperm morphology does not appear to influence blastocyst development or blastocyst morphology.[4] Even with severe teratozoospermia, microscopy can still detect the few sperm cells that have a "normal" morphology, allowing for optimal success rate.[4]
History
The first child born from a gamete micromanipulation was a child in Singapore born in April of 1989. [5]
The technique was developed by Gianpiero Palermo at the Vrije Universiteit Brussel, in the Center for Reproductive Medicine headed by Paul Devroey and Andre Van Steirteghem. [6]
The first activated embryo by ICSI was produced in 1990, but the first successful birth by ICSI took place on January 14, 1992. [7]
Procedure
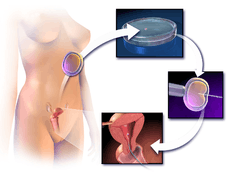
ICSI is generally performed following an in vitro fertilization procedure to extract one to several oocytes from a woman.
The procedure is done under a microscope using multiple micromanipulation devices (micromanipulator, microinjectors and micropipettes). A holding pipette stabilizes the mature oocyte with gentle suction applied by a microinjector. From the opposite side a thin, hollow glass micropipette is used to collect a single sperm, having immobilised it by cutting its tail with the point of the micropipette. The oocyte is pierced through the oolemma and directed to the inner part of the oocyte (cytoplasm). The sperm is then released into the oocyte. The pictured oocyte has an extruded polar body at about 12 o'clock indicating its maturity. The polar body is positioned at the 12 or 6 o'clock position, to ensure that the inserted micropipette does not disrupt the spindle inside the egg. After the procedure, the oocyte will be placed into cell culture and checked on the following day for signs of fertilization.
In contrast, in natural fertilization sperm compete and when the first sperm penetrates the oolemma, the oolemma hardens to block the entry of any other sperm. Concern has been raised that in ICSI this sperm selection process is bypassed and the sperm is selected by the embryologist without any specific testing. However, in mid-2006 the FDA cleared a device that allows embryologists to select mature sperm for ICSI based on sperm binding to hyaluronan, the main constituent of the gel layer (cumulus oophorus) surrounding the oocyte. The device provides microscopic droplets of hyaluronan hydrogel attached to the culture dish. The embryologist places the prepared sperm on the microdot, selects and captures sperm that bind to the dot. Basic research on the maturation of sperm shows that hyaluronan-binding sperm are more mature and show fewer DNA strand breaks and significantly lower levels of aneuploidy than the sperm population from which they were selected. A brand name for one such sperm selection device is PICSI.[8] A recent clinical trial showed a sharp reduction in miscarriage with embryos derived from PICSI sperm selection.[9]
'Washed' or 'unwashed' sperm may be used in the process.
Live birth rate are significantly higher with progesterone for luteal support in ICSI cycles.[10] Also, addition of a GNRH agonist for luteal support in ICSI cycles has been estimated to increase success rates,[10] by a live birth rate RD of +16% (95% confidence interval +10 to +22%).[11]
Using ultra-high magnification during sperm selection (with the technique being called IMSI) has no evidence of increased live birth or miscarriage rates compared to standard ICSI.[12]
Success or failure factors
One of the areas in which sperm injection can be useful is vasectomy reversal. However, potential factors that may influence pregnancy rates (and live birth rates) in ICSI include level of DNA fragmentation[13] as measured e.g. by Comet assay, advanced maternal age and semen quality.
Complications
There is some suggestion that birth defects are increased with the use of IVF in general, and ICSI specifically, though different studies show contradictory results. In a summary position paper, the Practice Committee of the American Society of Reproductive Medicine has said it considers ICSI safe and effective therapy for male factor infertility, but may carry an increased risk for the transmission of selected genetic abnormalities to offspring, either through the procedure itself or through the increased inherent risk of such abnormalities in parents undergoing the procedure.[14]
An online news story on MSNBC.com by Marilynn Marchione of the Associated Press, released on Saturday, May 5, 2012, discussed the risk of birth defects in ICSI versus natural conception or normal IVF; the following is a directly quoted segment of that release:
"Test-tube babies have higher rates of birth defects, and doctors have long wondered: Is it because of certain fertility treatments or infertility itself? A large new study from Australia suggests both may play a role.
Compared to those conceived naturally, babies that resulted from simple IVF, or in vitro fertilization — mixing eggs and sperm in a lab dish — had no greater risk of birth defects once factors such as the mother's age and smoking were taken into account.
However, birth defects were more common if treatment included injecting a single sperm into an egg, which is done in many cases these days, especially if male infertility is involved. About 10 percent of babies born this way had birth defects versus 6 percent of those conceived naturally, the study found." ..."
There is not enough evidence to say that ICSI procedures are safe in females with hepatitis B in regard to vertical transmission to the offspring, since the puncture of the oocyte can potentially avail for vertical transmission the offspring.[15]
Follow-up on fetus
In addition to regular prenatal care, prenatal aneuploidy screening based on maternal age, nuchal translucency scan and biomarkers is appropriate. However, biomarkers seem to be altered for pregnancies resulting from ICSI, causing a higher false-positive rate. Correction factors have been developed and should be used when screening for Down syndrome in singleton pregnancies after ICSI,[16] but in twin pregnancies such correction factors have not been fully elucidated.[16] In vanishing twin pregnancies with a second gestational sac with a dead fetus, first trimester screening should be based solely on the maternal age and the nuchal translucency scan as biomarkers are significantly altered in these cases.[16]
See also
- The Human Fertilisation and Embryology Authority (HFEA)
- Reproductive technology
- The Epigenome Network of Excellence (NoE)
References
- ↑ Hull et al., 1985
- ↑ Devroy et al., 1998
- ↑ Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ (2015). "Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection". JAMA 313 (3): 255–63. doi:10.1001/jama.2014.17985. PMID 25602996.
- 1 2 French DB, Sabanegh ES, Goldfarb J, Desai N (March 2010). "Does severe teratozoospermia affect blastocyst formation, live birth rate, and other clinical outcome parameters in ICSI cycles?". Fertil Steril 93 (4): 1097–1103. doi:10.1016/j.fertnstert.2008.10.051. PMID 19200957.
- ↑ https://books.google.com/books?id=KtMPBQAAQBAJ&pg=PA193&lpg=PA193&dq=SUZI+ng+%22april+1989%22&source=bl&ots=aFBDvWm-QT&sig=tuZcntK2Yp20VUtXXKVzNB8Yqu8&hl=en&sa=X&ved=0CB8Q6AEwAGoVChMImYCusrTYyAIVVM5jCh0nBQY2#v=onepage&q=SUZI%20ng%20%22april%201989%22&f=false
- ↑ Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. G Palermo, H Joris, P Devroey, AC Van Steirteghem - Lancet, 1992
- ↑ http://assets.cambridge.org/97811070/06478/excerpt/9781107006478_excerpt.pdf
- ↑ New Sperm Selection Technology for Assisted Reproductive Technology (ART) Cleared by FDA
- ↑ K.C. Worrilow, et al. "Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes- multicenter,double-blinded and randomized controlled trial." Human reproduction 28(2):306-14 (2013)
- 1 2 Van Der Linden, M.; Buckingham, K.; Farquhar, C.; Kremer, J. A. M.; Metwally, M. (2012). "Luteal phase support in assisted reproduction cycles". Human Reproduction Update 18 (5): 473. doi:10.1093/humupd/dms017.
- ↑ Kyrou, D.; Kolibianakis, E. M.; Fatemi, H. M.; Tarlatzi, T. B.; Devroey, P.; Tarlatzis, B. C. (2011). "Increased live birth rates with GnRH agonist addition for luteal support in ICSI/IVF cycles: A systematic review and meta-analysis". Human Reproduction Update 17 (6): 734–740. doi:10.1093/humupd/dmr029. PMID 21733980.
- ↑ Farquhar, C.; Rishworth, J. R.; Brown, J.; Nelen, W. L. M.; Marjoribanks, J. (2013). Brown, Julie, ed. "Assisted reproductive technology: an overview of Cochrane Reviews". The Cochrane Library 8: CD010537. doi:10.1002/14651858.CD010537.pub2. PMID 23970457.
- ↑ Speyer BE, Pizzey AR, Ranieri M, Joshi R, Delhanty JD, Serhal P (May 2010). "Fall in implantation rates following ICSI with sperm with high DNA fragmentation". Hum Reprod 25 (7): 1609–1618. doi:10.1093/humrep/deq116. PMID 20495207.
- ↑ Genetic considerations related to intracytoplasmic sperm injection (ICSI). Fertility and Sterility, Volume 86, Issue 5, Pages S103 - S105.
- ↑ Lutgens, S. P. M.; Nelissen, E. C. M.; Van Loo, I. H. M.; Koek, G. H.; Derhaag, J. G.; Dunselman, G. A. J. (2009). "To do or not to do: IVF and ICSI in chronic hepatitis B virus carriers". Human Reproduction 24 (11): 2676–2678. doi:10.1093/humrep/dep258. PMID 19625309.
- 1 2 3 Gjerris, Anne Catherine; Ann Tabor; Anne Loft; Michael Christiansen; Anja Pinborg (2012). "First trimester prenatal screening among women pregnant after IVF/ICSI". Human Reproduction Update 18 (4): 350–359. doi:10.1093/humupd/dms010. PMID 22523111.
Lancet. 1990 Mar 24;335(8691):722-3. Twin birth after subzonal insemination. Fishel S, Antinori S, Jackson P, Johnson J, Lisi F, Chiariello F, Versaci C.
Gordon, Ian R. Laboratory production of cattle embryos/I. Gordon. -- 2nd ed. 2003 ISBN 0-85199-666-3