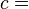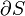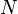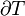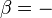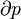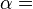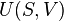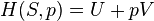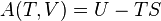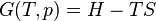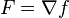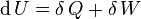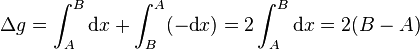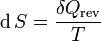Inexact differential
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
An inexact differential or imperfect differential is a specific type of differential used in thermodynamics to express the path dependence of a particular differential. It is contrasted with the concept of the exact differential in calculus, which can be expressed as the gradient of another function and is therefore path independent. Consequently, an inexact differential cannot be expressed in terms of its antiderivative for the purpose of integral calculations; i.e. its value cannot be inferred just by looking at the initial and final states of a given system.[1] It is primarily used in calculations involving heat and work because they are path functions, not state functions.
Definition
An inexact differential is commonly defined as a differential form dx where there is no corresponding function x such that: 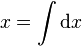 . More precisely, an inexact differential is a differential form that cannot be expressed as the differential of a function. In the language of calculus, for a given vector field F,
. More precisely, an inexact differential is a differential form that cannot be expressed as the differential of a function. In the language of calculus, for a given vector field F, 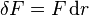 is an inexact differential if there is no function f such that
is an inexact differential if there is no function f such that
The fundamental theorem of calculus for line integrals requires path independence in order to express the values of a given vector field in terms of the partial derivatives of another function that is the multivariate analogue of the antiderivative. This is because there can be no unique representation of an antiderivative for inexact differentials since their variation is inconsistent along different paths. This stipulation of path independence is a necessary addendum to the fundamental theorem of calculus because in one-dimensional calculus there is only one path in between two points defined by a function.
First law of thermodynamics
Inexact differentials are known especially for their presence in the first law of thermodynamics:
The symbol δ instead of the plain d, which originated from the work of German mathematician Carl Gottfried Neumann[2] indicates that Q and W are path-dependent.
Internal energy U is a state function, meaning its change can be inferred just by comparing two different states of the system (not its transition path), which we can therefore indicate with U1 and U2. Since we can go from state U1 to state U2 either by providing heat Q = U2 − U1 or work W = U2 − U1, such a change of state does not identify uniquely the values of provided W and Q, but only the change in internal energy ΔU.
Examples
Although difficult to express mathematically, the inexact differential is very simple conceptually. There are many everyday examples that are much more relevant to inexact differentials in the actual context in which it is used.
Total distance
The easiest example is the difference between net distance and total distance. For example, in walking from Point A to Point B one covers a net distance B − A that is equal to the total distance. If one then returns to Point A, however, net distance is now 0 while total distance covered is 2*(B − A). This example captures the essential idea behind the inexact differential in one dimension.
Precisely, the differential of net distance is simply the exact one form 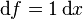 with corresponding function
with corresponding function 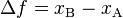 . It is exact because 1 has antiderivative x everywhere on the real line. On the other hand, the differential of total distance is the inexact one form
. It is exact because 1 has antiderivative x everywhere on the real line. On the other hand, the differential of total distance is the inexact one form 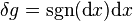 (i.e. the sign function). It is inexact because sgn(x) has antiderivative |x| which is not differentiable at x = 0. Therefore
(i.e. the sign function). It is inexact because sgn(x) has antiderivative |x| which is not differentiable at x = 0. Therefore 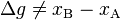 and instead we must look at the path dependence. In our example, in the first leg of the journey, sgn(dx) is 1 since x is increasing. In the second leg, sgn(dx) is −1 since x is decreasing. We can then evaluate the total distance as:
and instead we must look at the path dependence. In our example, in the first leg of the journey, sgn(dx) is 1 since x is increasing. In the second leg, sgn(dx) is −1 since x is decreasing. We can then evaluate the total distance as:
Heat and work
A fire requires heat, fuel, and an oxidizing agent. The energy required to overcome the activation energy barrier for combustion is transferred as heat into the system, resulting in changes to the system’s internal energy. In a process, the energy input to start a fire may comprise both work and heat, such as when one rubs tinder (work) and experiences friction (heat) to start a fire. The ensuing combustion is highly exothermic, which releases heat. The overall change in internal energy does not reveal the mode of energy transfer and quantifies only the net work and heat. The difference between initial and final states of the system’s internal energy does not account for the extent of the energy interactions transpired. Therefore, internal energy is a state function (i.e. exact differential), while heat and work are path functions (i.e. inexact differentials) because integration must account for the path taken.
Integrating factors
It is sometimes possible to convert an inexact differential into an exact one by means of an integrating factor. The most common example of this in thermodynamics is the definition of entropy:
In this case, δQ is an inexact differential, because its effect on the state of the system can be compensated by δW. However, when divided by the absolute temperature and when the exchange occurs at reversible conditions (therefore the rev subscript), it produces an exact differential: the entropy S is also a state function.
See also
- Closed and exact differential forms for a higher-level treatment
- Differential (mathematics)
- Exact differential
- Exact differential equation
- Integrating factor for solving non-exact differential equations by making them exact
- Conservative vector field
References
External links
- Inexact Differential – from Wolfram MathWorld
- Exact and Inexact Differentials – University of Arizona
- Exact and Inexact Differentials – University of Texas
- Exact Differential – from Wolfram MathWorld