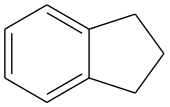
Indane
 | |
 | |
| Names | |
|---|---|
| IUPAC name
indane | |
| Other names | |
| Identifiers | |
| 496-11-7 | |
| ChEBI | CHEBI:37911 |
| ChEMBL | ChEMBL370687 |
| ChemSpider | 9903 |
| Jmol interactive 3D | Image |
| PubChem | 10326 |
| |
| |
| Properties | |
| C9H10 | |
| Molar mass | 118.176 g/mol |
| Appearance | Clear colorless liquid |
| Boiling point | 176.5 °C (349.7 °F; 449.6 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Indane or indan is a hydrocarbon petrochemical compound, with formula C9H10.
Derivatives
Derivatives include compounds such as 1-methyl-indane and 2-methyl-indane (where one methyl group is attached to the five carbon ring), 4-methyl-indane and 5-methyl-indane (where one methyl group is attached to the benzene ring), various dimethyl-indanes, and various pharmaceutical derivatives. Other derivatives can be obtained indirectly, e.g. the reaction of diethyl phthalate with ethyl acetate, using metallic sodium and ethanol as a catalyst. The reaction yields indanedione ethyl ester, which can react with the sodium ions yielding a salt. This can be reversed by adding an aqueous solution of hydrochloric acid.
Indane can also be converted in a catalytic reactor to other aromatics such as xylene.
Another derivative is 1,3-indandione.
Dr. David E. Nichols developed a family of indane derivatives that are empathogen-entactogens. They are very close derivatives of other empathogen-entactogens such as MDMA and MDA. However, they are not neurotoxic at therapeutic doses because the alpha-methyl group is bound and therefore cannot form a free radical. Examples include MDAI, MDMAI, and MDAT.[2] Nichols also created an indane isomer of amphetamine, 2-aminoindane, and an iodized derivative 5-IAI.
See also
References
- 1 2 Hawley, Gessner G. (1977). The Condensed Chemical Dictionary. Van Nostrand Reinhold Company. p. 464. ISBN 0-442-23240-3.
- ↑ Nichols DE, Brewster WK, Johnson MP, Oberlender R, Riggs RM. Nonneurotoxic tetralin and indan analogues of 3,4-(methylenedioxy)amphetamine (MDA). Journal of Medicinal Chemistry. 1990 Feb;33(2):703-10. PMID 1967651
3. https://pubchem.ncbi.nlm.nih.gov/compound/10326#section=Top