Hypofluorous acid
 Gas-phase structure | |
 | |
| Names | |
|---|---|
| IUPAC name
Hypofluorous acid | |
| Other names
Hydrogen hypofluorite Hydrogen fluorate(I) Fluoric(-I) acid Hydrogen monofluoroxygenate(0) | |
| Identifiers | |
| 14034-79-8 | |
| ChemSpider | 109936 |
| Jmol interactive 3D | Image |
| PubChem | 123334 |
| |
| |
| Properties | |
| HOF | |
| Molar mass | 36.0057 g mol−1 |
| Appearance | pale yellow liquid above −117 °C white solid below −117 °C |
| Melting point | −117 °C (−179 °F; 156 K) |
| Boiling point | < 0 °C decomposes at 0 °C |
| Structure | |
| Cs | |
| Related compounds | |
| Other cations |
lithium hypofluorite, LiOF |
| Related compounds |
hypochlorous acid, HOCl |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hypofluorous acid is the chemical compound with the formula HOF. It is an intermediate in the oxidation of water by fluorine, which produces hydrogen fluoride and oxygen. It is the only hypohalous acid that can be isolated as a solid. HOF is explosive, decomposing to oxygen and HF.[1] It was isolated in the pure form by fluorination of ice.
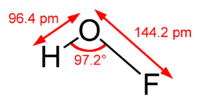
The compound has been characterized in the solid phase by X-ray crystallography[1] as a bent molecule with an angle of 101°. The O–F and O–H bond lengths are 144.2 and 96.4 picometres, respectively. The solid framework consists of chains with O–H---O linkages. The structure has also been analyzed in the gas phase, a state in which the H—O—F bond angle is slightly narrower (97.2°).
For fluorine, the only known oxoacid is hypofluorous acid, HOF. It can be made by passing F2 gas over ice at −40 °C, collecting the HOF gas, and condensing it:
- F2 + H2O → HOF + HF
It decomposes explosively at room temperature to form HF and O2:
- 2 HOF → 2 HF + O2
Hypofluorous acid in acetonitrile (generated in situ by passing gaseous fluorine through "wet" acetonitrile) serves as a highly electrophilic oxygen-transfer agent.[2][3] Treating phenanthroline with this reagent yielded the previously elusive 1,10-phenanthroline dioxide,[4] more than 50 years after the first unsuccessful attempt.[5]
This molecule is not very symmetric since the atoms are all different. It only has two symmetry operations: identity (E) and a mirror plane that goes through the three atoms. The assigned point group is Cs.
Hypofluorites
Hypofluorites are derivatives of OF−, which is the conjugate base of hypofluorous acid. One example is trifluoromethyl hypofluorite (CF3OF).
See also
- Hypochlorous acid, a related compound that is more technologically important but has not been obtained in pure form.
References
- 1 2 W. Poll, G. Pawelke, D. Mootz, E. H. Appelman (1988). "The Crystal Structure of Hypofluorous Acid : Chain Formation by O-H · · · O Hydrogen Bonds". Angew. Chem. Int. Ed. Engl. 27 (3): 392–3. doi:10.1002/anie.198803921.
- ↑ S. Rozen, M. Brand (1986). "Epoxidation of Olefins with Elemental Fluorine in Water/Acetonitrile Mixtures". Angew. Chem. Int. Ed. 25 (6): 554–5. doi:10.1002/anie.198605541.
- ↑ S. Dayan, Y. Bareket, S. Rozen (1999). "An efficient α-hydroxylation of carbonyls using the HOF·CH3CN complex". Tetrahedron 55 (12): 3657. doi:10.1016/S0040-4020(98)01173-9.
- ↑ S. Rozen, S. Dayan (1999). "At Last, 1,10-Phenanthroline-N,N'-dioxide, A New Type of Helicene, has been Synthesized using HOF·CH3CN". Angew. Chem. Int. Ed. 38 (23): 3471–3. doi:10.1002/(SICI)1521-3773(19991203)38:23<3471::AID-ANIE3471>3.0.CO;2-O.
- ↑ F. Linsker, R.L. Evans (1946). "Phenanthroline Di-N-oxides". J. Am. Chem. Soc. 68 (3): 403. doi:10.1021/ja01207a019.