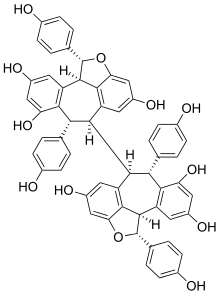
Hopeaphenol
 | |
| Names | |
|---|---|
| Other names
(-)-hopeaphenol | |
| Identifiers | |
| 17912-85-5 | |
| ChemSpider | 10277817 |
| Jmol interactive 3D | Image |
| PubChem | 495605 |
| |
| |
| Properties | |
| C56H42O12 | |
| Molar mass | 906.94 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hopeaphenol is a stilbenoid. It is a resveratrol tetramer. It has been first isolated from Dipterocarpaceae[1] like Shorea ovalis.[2] It has also been isolated from wines from North Africa.[3]
It shows an opposite effect to vitisin A on apoptosis of myocytes isolated from adult rat heart.[4]
See also
References
- ↑ The structure of hopeaphenol. P. Coggon, T. J. King and S. C. Wallwork, Chem. Commun. (London), 1966, pages 439-440, doi:10.1039/C19660000439
- ↑ The Isolation of Hopeaphenol, a Tetramer Stilbene, from Shorea ovalis Blume. Advances in Natural & Applied Sciences, January–April 2009, Volume 3, Issue 1, page 107 (abstract)
- ↑ Habiba Amira Guebailia, Kleopatra Chira, Tristan Richard, Teguiche Mabrouk, Aurélie Furiga, Xavier Vitrac, Jean-Pierre Monti, Jean-Claude Delaunay and Jean-Michel Mérillon (2006). "Hopeaphenol: The First Resveratrol Tetramer in Wines from North Africa". J. Agric. Food Chem. 54 (25): 9559–9564. doi:10.1021/jf062024g. PMID 17147446.
- ↑ Opposite Effects of Two Resveratrol (trans-3,5,4′-Trihydroxystilbene) Tetramers, Vitisin A and Hopeaphenol, on Apoptosis of Myocytes Isolated from Adult Rat Heart. Kazuhiko Seya, Kouta Kanemaru, Chiharu Sugimoto, Megumi Suzuki, Teruko Takeo, Shigeru Motomura, Haruo Kitahara, Masatake Niwa, Yoshiteru Oshima and Ken-Ichi Furukawa, JPET January 2009 vol. 328 no. 1 90-98, doi:10.1124/jpet.108.143172
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the Tuesday, July 07, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.