Homogeneity and heterogeneity

Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity in a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in one of these qualities.[1][2][3]
The words homogeneous and heterogeneous come from Medieval Latin homogeneus and heterogeneus, from Ancient Greek ὁμογενής (homogenēs) and ἑτερογενής (heterogenēs), from ὁμός (homos, “same”) and ἕτερος (heteros, “other, another, different”) respectively, followed by γένος (genos, “kind”); -ous is an adjectival suffix.[4]
The concepts are the same to every level of complexity, from atoms to populations of animals or people, and galaxies. Hence, an element may be homogeneous on a larger scale, compared to being heterogeneous on a smaller scale. This is known as an effective medium approach, or effective medium approximations.[5][6]
Heterogeneity
Heterogeneity is the state of being heterogeneous. It is the nature of opposition, or contrariety of qualities. It is diverse in kind or nature; composed of diverse parts, or resulting from differing causes. In general, a heterogeneous entity is composed of dissimilar parts, hence the constituents are of a different kind that can be distinguished from one another. The parts (or constituents) are connected, and of a conglomerate mass, and viewed in respect to the parts of which it is made up.[2][7]
Various disciplines understand heterogeneity, or being heterogeneous, in different ways. For example:
- In physics, it is understood as having physical properties that vary within the medium. For more information, please refer to article Homogeneity (physics).
- In chemistry, a heterogeneous material consists of either or both of a) multiple states of matter or b) hydrophilic and hydrophobic substances in one mixture; an example of the latter would be a mixture of water, octane, and silicone grease. Heterogeneous solids, liquids, and gases may be made homogeneous by melting, stirring, or by allowing time to pass for diffusion to distribute the molecules evenly. For example adding dye to water will create a heterogeneous solution at first, but will become homogeneous over time. Entropy allows for heterogeneous substances to become homogeneous over time.
- With information technology (see: Heterogeneous computing) it means a network comprising different types of computers, potentially with vastly differing memory sizes, processing power and even basic underlying architecture. Alternatively, a data resource with multiple types of formats..
- In sociology it may refer to a society or group that includes individuals of differing ethnicities, cultural backgrounds, sexes, or ages.
- Earth is a heterogeneous substance in many aspects. e.g. Rocks (geology) are inherently heterogeneous, usually occurring at the micro-scale and mini-scale.[5] Subsequently, in studying ore bodies and mineral deposits, hydrocarbon accumulation and reservoir characterization,[8] geoscientists always face heterogeneous and uncertain situation. Also in structural geology and many other earth-science fields, experts have to cope with heterogeneity in their investigations.
- In medicine and genetics, a genetic or allelic heterogeneous condition is one where the same disease or condition can be caused, or contributed to, by several factors. In the case of genetics, varying different genes or alleles.
- In cancer research, cancer cell heterogeneity is thought to be one of the underlying reasons that make treatment of cancer difficult.[9]
- In statistical meta-analysis, study heterogeneity is when multiple studies on an effect are actually measuring somewhat different effects due to differences in subject population, intervention, choice of analysis, experimental design, etc.; this can cause problems in attempts to summarize the meaning of the studies.
Homogeneity
Homogeneity is the state of being homogeneous. Pertaining to the sciences, it is a substance where all the constituents are of the same nature; consisting of similar parts, or of elements of the like nature. For example, homogeneous particles, homogeneous elements, homogeneous principles, or homogeneous bodies; or (algebra) possessing the same number of factors of a given kind as with a homogeneous polynomial.[3]
Chemistry
A heterogeneous mixture is a mixture of two or more compounds. Examples are: mixtures of sand and water or sand and iron filings, a conglomerate rock, water and oil, a salad, trail mix, and concrete (not cement).[10] During the sampling of heterogeneous mixtures of particles, the variance of the sampling error is generally non-zero. Gy's sampling theory [11] quantitatively defines the heterogeneity of a particle as:
 , the heterogeneity of the
, the heterogeneity of the  th particle of the population
th particle of the population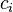 , the mass concentration of the property of interest in the
, the mass concentration of the property of interest in the  th particle of the population
th particle of the population ,the mass concentration of the property of interest in the population
,the mass concentration of the property of interest in the population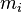 , the mass of the
, the mass of the  th particle in the population
th particle in the population , the average mass of a particle in the population
, the average mass of a particle in the population
Homogenization is the process of causing a heterogeneous mixture to become homogeneous, as is done with the making of homogenized milk.
Homogeneous and heterogeneous reactions
Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases. Reactions that take place on the surface of a catalyst of a different phase are also heterogeneous. A reaction between two gases or two miscible liquids is homogeneous. A reaction between a gas and a liquid, a gas and a solid or a liquid and a solid is heterogeneous.
A mixture can be determined to be homogeneous when everything is settled and equal, and the liquid, gas, object is one color or the same form. Various models have been proposed to model the concentrations in different phases. The phenomena to be considered are mass rates and reaction .
Spelling
The alternate spellings "homogenous" and "heterogenous" are commonly, but incorrectly, used to refer to the above. The word "homogenous" strictly speaking is a biological term which has largely been replaced by "homologous", though its use to mean "homogeneous" has seen a rise, since 2000, which makes it an "established variant".[12] "Heterogenous" meanwhile is a term primarily confined to pathology which refers to the property of an object in the body having its origin outside the body.[13]
See also
References
- ↑ Heterogeneous Mixtures, in chemistry, is where certain elements are unwillingly combined and, when given the option, will separates completely. "Webster's Revised Unabridged Dictionary (1913 + 1828)" (Part of this paragraph is public domain material copyright 1828 and 1913). Heterogeneity. The ARTFL Project, University of Chicago. September 2010. Retrieved 2010-09-10.
- 1 2 "Webster's Revised Unabridged Dictionary (1913 + 1828)" (Part of this paragraph is public domain material copyright 1828 and 1913). Heterogeneous. The ARTFL Project, University of Chicago. September 2010. Retrieved 2010-09-10.
- 1 2 "Webster's Revised Unabridged Dictionary (1913 + 1828)" (This is public domain material copyright 1828 and 1913). Homogeneous. The ARTFL Project, University of Chicago. September 2010. Retrieved 2010-09-10.
- ↑ http://www.oxforddictionaries.com/definition/english/heterogeneous
- 1 2 Guéguen,, Yves; Palciauskas, Victor (May 1994). Introduction to the physics of rocks. Princeton University Press. pp. 53–72 (Chapter 3). ISBN 978-0-691-03452-2.Google Books preview download available
- ↑ Shadrivov, Ilya V.; Kozyrev, AB; Van Der Weide, DW; Kivshar, YS (2008-11-24). "Nonlinear magnetic metamaterials" (Introduction section. Free PDF download). Optics Express 16 (25): 20266–71. Bibcode:2008OExpr..1620266S. doi:10.1364/OE.16.020266. PMID 19065165. Retrieved 2009-11-26.
- ↑ Heterogeneous Mixtures, in chemistry, is where certain elements are unwillingly combined and, when given the option, will separate. "Webster's Revised Unabridged Dictionary (1913 + 1828)" (Part of this paragraph is public domain material copyright 1828 and 1913). Heterogeneity. The ARTFL Project, University of Chicago. September 2010. Retrieved 2010-09-10.
- ↑ http://mmc2.igeofcu.unam.mx/cursos/gest/Articulos/Reservoir%20Caracterization/Multiple-point%20geostatistics%20a%20quantitative%20vehicle%20for%20integrating%20geologic%20analogs%20into%20multiple%20reservoir%20models.pdf
- ↑ Bhatia, Sangeeta; John V Frangioni; Robert M Hoffman; A John Iafrate; Kornelia Polyak (10 July 2012). "The challenges posed by cancer heterogeneity". Nature Biotechnology 30: 604–610. doi:10.1038/nbt.2294.
- ↑ Gamow, George (April 1967). "Chapter VI, "Descending Staircase"". One Two Three... Infinity (Mass market paperback) (Bantam Science and Mathematics, 5th printing ed.). Bantam. p. 117.
[Clam chowder] represents a nice example of what is known as a heterogeneous material.
- ↑ Gy, P. (1979), Sampling of Particulate Materials: Theory and Practice, Elsevier: Amsterdam, 431 pp.
- ↑ http://www.oxforddictionaries.com/definition/english/homogeneous
- ↑ http://grammarist.com/usage/heterogeneous-heterogenous/
External links
| Look up homogeneity, heterogeneity, homogeneous, or heterogeneous in Wiktionary, the free dictionary. |
- The following cited pages in this book cover the meaning of "homogeneity" across disciplines Morris, Christopher G. (1992). Academic Press Dictionary of Science and Technology. pp. 1039, 1040. ISBN 0-12-200400-0..
