Altretamine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
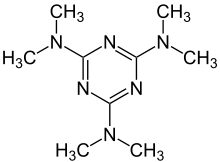
N2,N2,N4,N4,N6,N6-hexamethyl-1,3,5-triazine-2,4,6-triamine | |
| Clinical data | |
| Trade names | Hexalen |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a601200 |
| Licence data | US FDA:link |
| Pregnancy category | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 94% |
| Biological half-life | 4.7-10.2 hours |
| Identifiers | |
| CAS Number |
645-05-6 |
| ATC code | L01XX03 |
| PubChem | CID 2123 |
| IUPHAR/BPS | 7112 |
| DrugBank |
DB00488 |
| ChemSpider |
2038 |
| UNII |
Q8BIH59O7H |
| KEGG |
D02841 |
| ChEBI |
CHEBI:24564 |
| ChEMBL |
CHEMBL1455 |
| Chemical data | |
| Formula | C9H18N6 |
| Molar mass | 210.28 g/mol |
| |
| |
| (verify) | |
Altretamine (also hexalen) is an antineoplastic agent. It was approved by the FDA in 1990.
Uses
It is used to treat refractory ovarian cancer.
It is not considered a first-line treatment,[1] but it can be useful as salvage therapy.[2] It also has the advantage of being less toxic than other drugs used for treating refractory ovarian cancer.[3]
Mechanism
The precise mechanism by which altretamine exerts its anti-cancer effect is unknown but it is classified by MeSH as an alkylating antineoplastic agent.[4] This unique structure is believed to damage tumor cells through the production of the weakly alkylating species formaldehyde, a product of CYP450-mediated N-demethylation. Administered orally, altretamine is extensively metabolized on first pass, producing primarily mono- and didemethylated metabolites. Additional demethylation reactions occur in tumor cells, releasing formaldehyde in situ before the drug is excreted in the urine. The carbinolamine (methylol) intermediates of CYP450-mediated metabolism also can generate electrophilic iminium species that are capable of reacting covalently with DNA guanine and cytosine residues as well as protein. Iminium-mediated DNA cross-linking and DNA-protein interstrand cross-linking, mediated through both the iminium intermediate and formaldehyde, have been demonstrated, although the significance of DNA cross-linking on altretamine antitumor activity is uncertain.[5]
Side effects
Side effects include nausea, vomiting, anemia and peripheral sensory neuropathy.[6]
Interactions
Combination with pyridoxine (vitamin B6) decreases neurotoxicity but has been found to reduce the effectiveness of an altretamine/cisplatin regime.[7] MAO inhibitor can cause severe orthostatic hypotension when combined with altretamine; and cimetidine can increase its elimination half-life and toxicity.[6]
See also
References
- ↑ Keldsen N, Havsteen H, Vergote I, Bertelsen K, Jakobsen A (2003). "Altretamine (hexamethylmelamine) in the treatment of platinum-resistant ovarian cancer: a phase II study". Gynecol. Oncol. 88 (2): 118–22. doi:10.1016/S0090-8258(02)00103-8. PMID 12586589.
- ↑ Chan JK, Loizzi V, Manetta A, Berman ML (2004). "Oral altretamine used as salvage therapy in recurrent ovarian cancer". Gynecol. Oncol. 92 (1): 368–71. doi:10.1016/j.ygyno.2003.09.017. PMID 14751188.
- ↑ Malik IA (2001). "Altretamine is an effective palliative therapy of patients with recurrent epithelial ovarian cancer". Jpn. J. Clin. Oncol. 31 (2): 69–73. doi:10.1093/jjco/hye012. PMID 11302345.
- ↑ Damia G, D'Incalci M (1995). "Clinical pharmacokinetics of altretamine". Clinical pharmacokinetics 28 (6): 439–48. doi:10.2165/00003088-199528060-00002. PMID 7656502.
- ↑ "Foy`s principles of Medical chemistry", edited by Thomas L. Lemke, sixth edition, 2008, 1162 pages, ISBN 978-0-7817-6879-5.
- 1 2 Drugs.com: Altretamine Monograph
- ↑ Wiernik, P. H.; Yeap, B.; Vogl, S. E.; Kaplan, B. H.; Comis, R. L.; Falkson, G.; Davis, T. E.; Fazzini, E.; Cheuvart, B.; Horton, J. (1992). "Hexamethylmelamine and low or moderate dose cisplatin with or without pyridoxine for treatment of advanced ovarian carcinoma: A study of the Eastern Cooperative Oncology Group". Cancer investigation 10 (1): 1–9. doi:10.3109/07357909209032783. PMID 1735009.
| ||||||||||||||||||||||||||||||||||||||