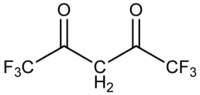
Hexafluoroacetylacetone
 | |
| Names | |
|---|---|
| IUPAC name
1,1,1,5,5,5-Hexafluoro-2,4-pentanedione | |
| Other names
Hexafluoroacetylacetone, HfacH | |
| Identifiers | |
| 1522-22-1 | |
| ChemSpider | 21106446 |
| Jmol interactive 3D | Image |
| PubChem | 24874462 |
| |
| |
| Properties | |
| C5H2F6O2 | |
| Molar mass | 208.06 g/mol |
| Appearance | colourless liquid |
| Density | 1.47 g/mL |
| Boiling point | 70 to 71 °C (158 to 160 °F; 343 to 344 K) |
| organic solvents | |
| Hazards | |
| Main hazards | toxic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hexafluoroacetylacetone is the chemical compound with the formula CF3C(O)CH2C(O)CF3 (often abbreviated as hfac). This colourless liquid is a ligand precursor and a reagent used in MOCVD. Complexes of the conjugate base, often called Hfac−, exhibit enhanced volatility and Lewis acidity relative to analogous complexes derived from acetylacetone.
This organofluorine compound was first prepared by the condensation of ethyl ester of trifluoroacetic acid and 1,1,1-trifluoroacetone.[1] It has been investigated as an etchant for copper and its complexes, such as Cu(Hfac)(trimethylvinylsilane) have been employed as precursors in microelectronics.[2]
Being highly electrophilic, hfac hydrates in water to give the tetraol.[3]
References
- ↑ Henne, Albert L.; Newman, Melvin S.; Quill, Laurence L.; Staniforth, Robert A. (1947). "Alkaline condensation of fluorinated esters with esters and ketones". Journal of the American Chemical Society 69 (7): 1819–20. doi:10.1021/ja01199a075.
- ↑ Mark J. Hampden-Smith, Toivo T. Kodas (1995). "Chemical vapour deposition of copper from (hfac)CuL compounds". Polyhedron 14 (6): 699–732. doi:10.1016/0277-5387(94)00401-Y.
- ↑ Aygen, S.; van Eldik, R. (1989). "A Spectroscopic and Mechanistic Study of the Enolization and Diol Formation of Hexafluoroacetylacetone in the Presence of Water and Alcohol". Chem. Ber. 122: 315. doi:10.1016/S0022-328X(00)90307-3.
This article is issued from Wikipedia - version of the Tuesday, February 02, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.