Hepoxilin
 | |
| Names | |
|---|---|
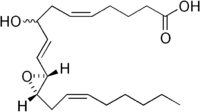
| IUPAC name
(5E,9E)-8-hydroxy-10-[3-[(E)-oct-2-enyl] -2-oxiranyl]deca-5,9-dienoic acid | |
| Other names
HXA3 | |
| Identifiers | |
| 94161-11-2 | |
| Jmol interactive 3D | Image |
| PubChem | 5353666 |
| |
| Properties | |
| C20H32O4 | |
| Molar mass | 336.47 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hepoxilins (HxA3 and HxB3) are nonclassic eicosanoid hormones involved in inflammation.
History
Hepoxilins were identified and named in Canada in 1984 by CR Pace-Asciak and JM Martin.[1]
Biochemistry
They derive from arachidonic acid via oxidation by the enzyme 12-lipoxygenase. Hepoxilins are differentiated from closely related eicosanoids, the leukotrienes and the lipoxins, in that hepoxilins have no conjugated double bonds. Corresponding trioxlins A4 and B4 are formed by the same pathway from EPA [2] Two, more recently described hepoxins, 11(S)-hydroxy-14(S),15(S)-epoxy-5(Z),8(Z),12(E)-eicosatrienoic acid and 13(R)-hydroxy-14(S),15(S)-epoxy-5(Z),8(Z),11(Z)-eicosatrienoic acid, termed respectively 14,15-HXA3 and 14,15-HXB3 are made by 15-lipoxygenase-1 with 15(S)-hydroperoxy-eicosatetraenoic acid as an intermediate; 14,15-HXA3 may then be further metabolized by glutathione transferases to 11(S),15(S)-dihydroxy-14(R)-glutathionyl-(5Z),8(Z),12(E)eicosatrienoic acid (14,15-HXA3C) which is then further metabolized to 11(S),15(S)-dihydroxy-14(R)-cysteinyl-glycyl-(5Z),8(Z),12(E)eicosatrienoic acid (14,15-HXA3D).[3] The latter two hepoxilins are analogous to eoxins and leukotrienes and presumed to share the same glutatione transferase and peptidase pathways in their formation (see 15-Hydroxyicosatetraenoic acid).
Physiological effect
In the skin, Hx are pro-inflammatory, but in neutrophils they are anti-inflammatory.[4] Hx are potent insulin secretagogues.[2] One hepoxilin, HepA3, mediates neutrophil migration across the intestines.[5] Hepoxilins are also produced in the brain.[6] Cells under oxidative stress secrete HX3, which in turn upregulates peroxidase, decreasing oxidative stress. This is proposed to constitute a compensatory defense response to protect the vitality and functionality of the cell.[7]
References
- ↑ Pace-Asciak CR, Martin JM (1984). "Hepoxilin, a new family of insulin secretagogues formed by intact rat pancreatic islets". Prostaglandins, leukotrienes, and medicine 16 (2): 173–80. doi:10.1016/0262-1746(84)90069-6. PMID 6396652.
- 1 2 Pace-Asciak CR (1986). "Formation of hepoxilin A4, B4 and the corresponding trioxilins from 12(S)-hydroperoxy-5,8,10,14,17-icosapentaenoic acid". Prostaglandins, leukotrienes, and medicine 22 (1): 1–9. doi:10.1016/0262-1746(86)90017-X. PMID 3012585.
- ↑ Lipids 4:69-79, 2011
- ↑ Christie, William W. (2006). "LEUKOTRIENES AND LIPOXINS: Chemistry and Biology". Retrieved January 4, 2007.
- ↑ Randall J. Mrsny, Andrew T. Gewirtz, Dario Siccardi, Tor Savidge , Bryan P. Hurley , James L. Madara, and Beth A. McCormick (2004). "Identification of hepoxilin A3 in inflammatory events: A required role in neutrophil migration across intestinal epithelia". Proceedings of the National Academy of Sciences of the United States of America 101 (19): 7421–6. doi:10.1073/pnas.0400832101. PMC 409934. PMID 15123795. Retrieved January 4, 2007.
- ↑ Piomelli, Daniele (2000). "Arachidonic Acid". Neuropsychopharmacology: The Fifth Generation of Progress (New York: Chapman & Hall). ISBN 0-412-10951-4. Retrieved 2006-03-03.
- ↑ M.P. Zafiriou; et al. (October–November 2007). "Prostaglandins, Leukotrienes and Essential Fatty Acids : Biological role of hepoxilins: Upregulation of phospholipid hydroperoxide glutathione peroxidase as a cellular response to oxidative stress?". Prostaglandins, Leukotrienes and Essential Fatty Acids 77 (3–4): 209–215. doi:10.1016/j.plefa.2007.08.007. PMID 17997296. Retrieved 2007-12-04.