Hemetsberger indole synthesis
The Hemetsberger indole synthesis (also called the Hemetsberger–Knittel synthesis) is a chemical reaction that thermally decomposes a 3-aryl-2-azido-propenoic ester into an indole-2-carboxylic ester.[1][2]
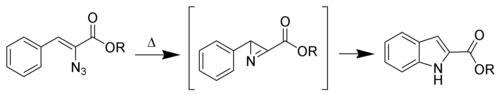
Yields are typically above 70%. However, this is not a popular reaction, due to the lack of stability and difficulty in synthesizing the starting material.
Reaction mechanism
The mechanism is unknown. However, aziridine[3] intermediates have been isolated. The mechanism is postulated to proceed via a nitrene intermediate.[4]
References
- ↑ Hemetsberger, H.; Knittel, D. (1972). "Synthese und Thermolyse von α-Azidoacrylestern". Monatsh. Chem. 103: 194. doi:10.1007/BF00912944.
- ↑ Gribble, G. W. (2000). "Recent developments in indole ring synthesis-methodology and applications". J. Chem. Soc., Perkin Trans. (7): 1045–1075. doi:10.1039/a909834h.
- ↑ Gilchrist, T. L. (2001). "Activated 2H-Azirines as Dienophiles and Electrophiles" (PDF). Aldrichimica Acta 34 (2): 51.
- ↑ Knittel, D. (1985). "Verbesserte Synthese von α-Azidozimtsäure-estern und 2H-Azirinen". Synthesis 1985 (2): 186–188. doi:10.1055/s-1985-31149.
This article is issued from Wikipedia - version of the Monday, May 25, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.