Axial chirality
Axial chirality is a special case of chirality in which a molecule does not possess a stereogenic center (the most common form of chirality in organic compounds) but an axis of chirality – an axis about which a set of substituents is held in a spatial arrangement that is not superposable on its mirror image. Axial chirality is most commonly observed in atropisomeric biaryl compounds wherein the rotation about the aryl-aryl bond is restricted, for example, biphenyl, binaphthyls, e.g., 1,1'-bi-2-naphthol, and certain dihydroanthracenone compounds.[1] Certain allene compounds also display axial chirality. The enantiomers of axially chiral compounds are usually given the stereochemical labels Ra and Sa.[2] The designations are based on the same Cahn-Ingold-Prelog priority rules used for tetrahedral stereocenters. The chiral axis is viewed end-on and the two "near" and two "far" substituents on the axial unit are ranked.
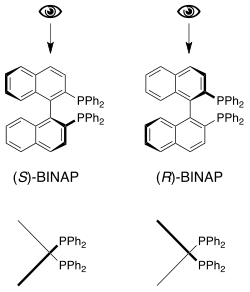
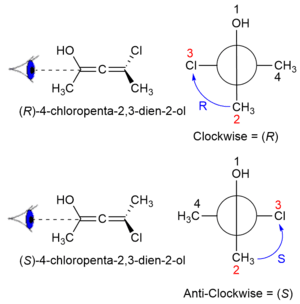
This property can also be called helicity, since the axis of the structure has a helical, propeller, or screw-shaped geometry. P (plus) or Δ is a right-handed helix, where M (minus) or Λ is a left-handed helix.[3][4] The P/M or Δ/Λ terminology is used particularly for molecules that actually resemble a helix, such as hexahelicene. It can also be applied to other structures having axial chirality by considering the helical orientation of the "front" vs "back" Cahn–Ingold–Prelog rankings.
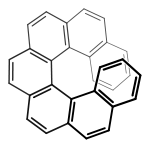
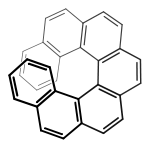
External links
References
- ↑ Absolute stereochemistry of fungal metabolites: Icterinoidins A1 and B1, and atrovirins B1 and B2 Melvyn Gill and Peter M. Morgan Arkivoc (RI-1154C)2004 Online article
- ↑ Compendium of Chemical Terminology, axial chirality
- ↑ Compendium of Chemical Terminology, helicity
- ↑ VLU: Additional Chirality Elements - Chemgapedia
| ||||||||||||||||||||||