Hantzsch pyridine synthesis
| Hantzsch pyridine synthesis | |
|---|---|
| Named after | Arthur Rudolf Hantzsch |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | hantzsch-dihydropyridine-synthesis |
| RSC ontology ID | RXNO:0000268 |
The Hantzsch pyridine synthesis or Hantzsch dihydropyridine synthesis is a multi-component organic reaction between an aldehyde such as formaldehyde, 2 equivalents of a β-keto ester such as ethyl acetoacetate and a nitrogen donor such as ammonium acetate or ammonia.[1] The initial reaction product is a dihydropyridine which can be oxidized in a subsequent step to a pyridine. The driving force for this second reaction step is aromatization. This reaction was reported in 1881 by Arthur Rudolf Hantzsch.
A 1,4-dihydropyridine dicarboxylate is also called a 1,4-DHP compound or a Hantzsch compound. These compounds are an important class of calcium channel blockers and as such commercialized in for instance nifedipine, amlodipine or nimodipine.
The reaction has been demonstrated to proceed in water as reaction solvent and with direct aromatization by ferric chloride, Manganese Dioxide or potassium permanganate in a one-pot synthesis.[2]
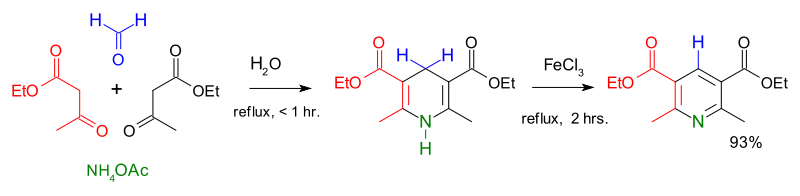
The Hantzsch dihydropyridine synthesis is found to benefit from microwave chemistry.[3]
Knoevenagel–Fries modification
The Knoevenagel–Fries modification allows for the synthesis of unsymmetrical pyridine compounds.[4]
References
- ↑ Hantzsch, A. (1881). "Condensationprodukte aus Aldehydammoniak und Ketonartigen Verbindungen". Chemische Berichte 14 (2): 1637–8. doi:10.1002/cber.18810140214.
- ↑ Xia, J. J.; Wang, G. W. (2005). "One-Pot Synthesis and Aromatization of 1,4-Dihydropyridines in Refluxing Water". Synthesis 2005 (14): 2379–83. doi:10.1055/s-2005-870022.
- ↑ van den Eynde, J. J.; Mayence, A. (2003). "Synthesis and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview" (PDF). Molecules 8 (4): 381–91. doi:10.3390/80400381.
- ↑ Knoevenagel, E.; Fries, A. (1898). "Synthesen in der Pyridinreihe. Ueber eine Erweiterung der Hantzsch'schen Dihydropyridinsynthese". Berichte der Deutschen Chemischen Gesellschaft 31 (1): 761–7. doi:10.1002/cber.189803101157.