Fluoxymesterone
 | |
| Systematic (IUPAC) name | |
|---|---|
|
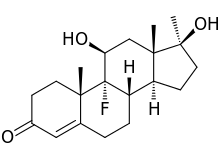
9-fluoro-11,17-dihydroxy-10,13,17-trimethyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682690 |
| Pregnancy category |
|
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% Oral |
| Metabolism | Hepatic |
| Biological half-life | 9.5 hours |
| Excretion | urine |
| Identifiers | |
| CAS Number |
76-43-7 |
| ATC code | G03BA01 |
| PubChem | CID 6446 |
| IUPHAR/BPS | 2861 |
| DrugBank |
DB01185 |
| ChemSpider |
6205 |
| UNII |
9JU12S4YFY |
| KEGG |
D00327 |
| ChEBI |
CHEBI:5120 |
| ChEMBL |
CHEMBL1445 |
| Chemical data | |
| Formula | C20H29FO3 |
| Molar mass | 336.441 g/mol |
| |
| |
| (verify) | |
Fluoxymesterone (trade name Halotestin) is an anabolic steroid with strong androgenic properties that has been used in the treatment of male hypogonadism, delayed puberty in males, and in the treatment of breast neoplasms in women. It is approximately 5 times as potent as testosterone.[1] The antitumor activity of fluoxymesterone appears related to reduction or competitive inhibition of prolactin receptors or estrogen receptors or production. Dissimilar to testosterone, halotestin has a 100% bioavailability, as the methylation of the 17α-position in halotestin inhibits hepatic metabolism by enzymatic oxidation of 17β-hydroxyl, allowing its absorption into the bloodstream for transport around the body. While there are many legitimate pharmaceutical applications of halotestin, it is also abused, leading to the development of analytical techniques by which halotestin doping can be identified.
Like many C-17 alpha alkylated steroids, fluoxymesterone has poor binding to the androgen receptor. Even so, its actions are mediated by the androgen receptor, most-likely due to its prolonged plasma half-life which is approximately 9.2 hours.[2]
Analytical testing for steroid abuse in professional athletics
Detection of halotestin and other such illegal anabolic steroids in sports is achieved by GS-MS identification of urinary excreted anabolic steroids and their metabolites. In a test for halotestin, a dry residue obtained from a urine sample is dissolved in dimethylformamide and a sulfur trioxide-pyridine complex and is heated with 1% potassium carbonate solution. Halotestin and many of its metabolites contain two polar hydroxyl groups, leading to intermolecular hydrogen bonding that increases their boiling point and reduces volatility. In order to attain a gaseous sample for GC-MS, the products of hydrolysis are extracted, dissolved in methanol and derivatised to form volatile trimethylsilyl (TMS) esters by adding N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA) and trimethylsilylimidazole (TMSImi).[3]
Synthesis
Step One: The first step in the synthesis of fluoxymesterone is the microbiological oxidation of commercially available androstenedione (1.11) by Actinomyces; this introduces a hydroxyl group to the 11α-position (1.12), which is then oxidised to a ketone using Jones’ reagent, yielding the 3,11,17-triketone, adrenosterone (1.13). Pyrrolidine then reacts to form an enamine (1.14) by reaction with the 3α-keto group, protecting it from alkylation in a subsequent step. The regioselectivity of pyrrolidine for reaction at the 3α-position occurs inherently in the structure of adrenosterone, due to the position of the sterically bulky methyl groups. In subsequent steps, alkylation of the 17-keto group (1.14) using Grignard reagent, addition of hydride at the 11-position (1.15) and regeneration of the protected 3-keto group yields the starting material (1.16) for the final steps of the fluoxymesterone synthesis. This involves more standard synthetic transformations.
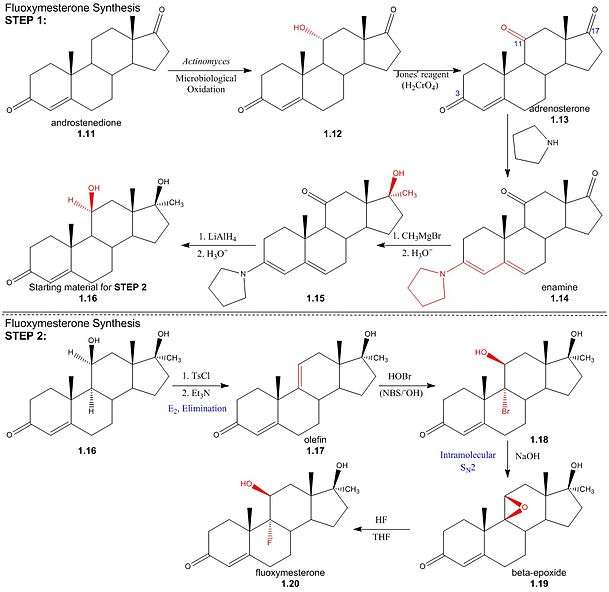
StepTwo: The 11α-hydroxyl of the starting material (1.16) is sulfonylated by p-toluenesulfonyl chloride; addition of trimethylamine (base) deprotonates the 11α-carbon, yielding an (E2) elimination of tosylate (pka - 5) to give olefin (1.17). Stereospecificity of reaction between olefin and hypobromous acid (HOBr) in base, N-bromosuccinimide (NBS), is determined by the formation of a bromonium intermediate; the electrophilic bromonium cation approaches the ring’s less sterically hindered α-face and is attacked by the π-electron density of the alkene. The hydroxide ion then attacks from above the ring (β-face) at the 11-carbon, resulting in a structure (1.18) by the stereospecific addition of hydroxyl and bromine across the double bond. Addition of sodium hydroxide results in deprotonation of the 11α-hydroxyl, and the subsequent structure undergoes an intramolecular SN2 epoxy ring formation. The epoxy ring of the β-epoxide (1.19) is protonated to give an oxironium ion intermediate. In a concerted process, fluoride attacks the ring’s α-face from below, as one of the two oxygen-carbon bonds is broken on the opposite face; hence regenerating the 11α-hydroxyl trans to the fluorine substituent. The resulting structure (1.20) is the androgenic steroid, fluoxymesterone.
References
- ↑ Dr. K.V. Sastry (2008). Endocrinology and Reproductive Biology. Page 150. ISBN 81-7133-777-5.
- ↑ Seth Roberts (2009). Anabolic Pharmacology.
- ↑ Schänzer, Willi; Opfermann, Georg; Donike, Manfred (1992-11-01). "17-Epimerization of 17α-methyl anabolic steroids in humans: metabolism and synthesis of 17α-hydroxy-17β-methyl steroids". Steroids 57 (11): 537–550. doi:10.1016/0039-128X(92)90023-3.
Further reading
- Daniels, R. C. (February 1, 2003). The Anabolic Steroid Handbook. Richard C Daniels. p. 80. ISBN 0-9548227-0-6.
| ||||||||||||||||||||||||||||||||||||||