Halobacterium salinarum
| Halobacterium salinarum | |
|---|---|
| Scientific classification | |
| Domain: | Archaea |
| Kingdom: | Euryarchaeota |
| Phylum: | Euryarchaeota |
| Class: | Halobacteria |
| Order: | Halobacteriales |
| Family: | Halobacteriaceae |
| Genus: | Halobacterium |
| Species: | H. salinarium |
| Binomial name | |
| Halobacterium salinarium corrig. (Harrison and Kennedy 1922) Elazari-Volcani 1957 | |
| Synonyms | |
|
Pseudomonas salinaria Harrison and Kennedy 1922 | |
Halobacterium salinarum is an extremely halophilic marine Gram-negative obligate aerobic archaeon. Despite its name, this microorganism is not a bacterium, but rather a member of the domain Archaea.[1] It is found in salted fish, hides, hypersaline lakes, and salterns. As these salterns reach the minimum salinity limits for extreme halophiles, their waters become purple or reddish color due to the high densities of halophilic Archaea.[1] H. salinarum has also been found in high-salt food such as salt pork, marine fish, and sausages. The ability of H. salinarum to survive at such high salt concentrations has led to its classification as an extremophile.
Cell morphology and metabolism
Halobacteria are single-celled, rod-shaped microorganisms that are among the most ancient forms of life and appeared on Earth billions of years ago. . The membrane consists of a single lipid monolayer surrounded by an S-layer.[2] The S-layer is made of a cell-surface glycoprotein, which accounts for approximately 50% of the cell surface proteins.[3] These proteins form a lattice in the membrane. Sulfate residues are abundant on the glycan chains of the glycoprotein, giving it a negative charge. The negative charge is believed to stabilize the lattice in high-salt conditions.[4]
Amino acids are the main source of chemical energy for H. salinarum, particularly arginine and aspartate, though they are able to metabolize other amino acids, as well.[2] H. salinarum have been reported to not be able to grow on sugars, and therefore need to encode enzymes capable of performing gluconeogenesis to create sugars. Although "H. salinarum" is unable to catabolize glucose, the transcription factor TrmB has been proven to regulate the gluconeogenic production of sugars found on the S-layer glycoprotein.
Adaptation to extreme conditions
High salt
To survive in extremely salty environments, this archaeon—as with other halophilic Archaeal species—utilizes compatible solutes (in particular potassium chloride) to reduce osmotic stress.[5] Potassium levels are not at equilibrium with the environment, so H. salinarum expresses multiple active transporters which pump potassium into the cell.[2] At extremely high salt concentrations protein precipitation will occur. To prevent the salting out of proteins, H. salinarum encodes mainly acidic proteins. The average isoelectric point of H. salinarum proteins is 4.9. These highly acidic proteins are overwhelmingly negative in charge and are able to remain in solution even at high salt concentrations.[1]
Low oxygen and photosynthesis
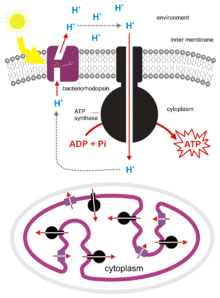
H. salinarum can grow to such densities in salt ponds that oxygen is quickly depleted. Though it is an obligate aerobe, it is able to survive in low-oxygen conditions by utilizing light-energy. H. salinarum express the membrane protein bacteriorhodopsin[8] which acts as a light-driven proton pump. It consists of two parts, the 7-transmembrane protein, bacterioopsin, and the light-sensitive cofactor, retinal. Upon absorption of a photon, retinal changes conformation, causing a conformational change in the bacterioopsin protein which drives proton transport.[9] The proton gradient which is formed can then be used to generate chemical energy by ATP synthase.
To obtain more oxygen H. salinarum produce gas vesicles, which allow them to float to the surface where oxygen levels are higher and more light is available.[10] These vesicles are complex structures made of proteins encoded by at least 14 genes.[11] Gas vesicles were first discovered in H. salinarum in 1967.[12]
UV protection
There is little protection from the Sun in salt ponds, so H. salinarum are often exposed to high amounts of UV radiation. To compensate, they have evolved a sophisticated DNA repair mechanism. The genome encodes DNA repair enzymes homologous to those in both bacteria and eukaryotes.[1] This allows H. salinarum to repair damage to DNA faster and more efficiently than other organisms and allows them to be much more UV tolerant.
H. salinarum is responsible for the bright pink or red appearance of the Dead Sea and other bodies of salt water. This red color is due primarily to the presence of bacterioruberin, a 50 carbon carotenoid pigment present within the membrane of H. salinarum. The primary role of bacterioruberin in the cell is to protect against DNA damage incurred by UV light.[13] This protection is not, however, due to the ability of bacterioruberin to absorb UV light. Bacterioruberin protects the DNA by acting as an antioxidant, rather than directly blocking UV light.[14] It is able to protect the cell from reactive oxygen species produced from exposure to UV by acting as a target. The bacterioruberin radical produced is less reactive than the initial radical, and will likely react with another radical, resulting in termination of the radical chain reaction.[15]
Genome
Whole genome sequences are available for two strains of H. salinarum, NRC-1[2] and R1.[16] The Halobacterium sp. NRC-1 genome consists of 2,571,010 base pairs on one large chromosome and two mini-chromosomes. The genome encodes 2,360 predicted proteins.[2] The large chromosome is very G-C rich (68%).[17] High GC-content of the genome increases stability in extreme environments. Whole proteome comparisons show the definite archaeal nature of this halophile with additional similarities to the Gram-positive Bacillus subtilis and other bacteria.
H. salinarum as a model organism
H. salinarum is as easy to culture as E. coli and serves as an excellent model system. Methods for gene replacement and systematic knockout have been developed,[18] so H. salinarum is an ideal candidate for the study of archaeal genetics and functional genomics.
H. salinarum for hydrogen production
Hydrogen production using H. salinarium coupled to a hydrogenase donor like E. coli are reported in literature.[19]
Oldest DNA ever recovered
A sample of a close genetic relative of H. salinarum encapsulated in salt has allowed for the recovery of DNA fragments estimated at 121 million years old. Oddly, the material had been also recovered earlier, but it proved to be so similar to the modern descendants that scientists had believed the earlier samples were contaminated.
Scientists have previously recovered similar genetic material from the Michigan Basin, the same region where the latest discovery was made. But that DNA, discovered in a salt-cured buffalo hide in the 1930s, was so similar to that of modern microbes that many scientists believed the samples had been contaminated.[20] The curing salt had been derived from a mine in Saskatchewan, the site of the most recent sample described by Jong Soo Park of Dalhousie University in Halifax, Nova Scotia, Canada.[21]
Russell Vreeland of Ancient Biomaterials Institute of West Chester University in Pennsylvania, USA, performed an analysis of all known halopathic bacteria, which yielded the finding that Park's bacteria contained six segments of DNA never seen before in the halopaths. Vreeland also tracked down the buffalo skin and determined that the salt came from the same mine as Park's sample. He has also discovered an even older halopath estimated at 250 million years old in New Mexico.[22]
References
- 1 2 3 4 DasSarma, S., Extreme Microbes, Am Sci, 2007, 95(3):224–231.
- 1 2 3 4 5 Ng, W.V., et al., Genome sequence of Halobacterium species NRC-1, Proc Natl Acad Sci USA, 2000, 97(22):12176–12181.
- ↑ Mescher, M.F., Strominger, J.L., Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium, J Biol Chem, 1976, 251(7):2005–2014.
- ↑ Sara, M., Sleytr, U.B., S-layer proteins. J Bacteriol. 2000, 182(4):859–68.
- ↑ Pérez-Fillol, M., Rodrguez-Valera, F., Potassium ion accumulation in cells of different halobacteria, Microbiologia, 1986, 2(2):73–80.
- ↑ Nicholls D. G., Ferguson S, J. (1992). Bioenergetics 2 (2nd ed.). San Diego: Academic Press. ISBN 9780125181242.
- ↑ Stryer, Lubert (1995). Biochemistry (fourth ed.). New York – Basingstoke: W. H. Freeman and Company. ISBN 978-0716720096.
- ↑ Oesterhelt, D., Functions of a new photoreceptor membrane, Proc Natl Acad Sci USA, 1973, 70(10):2853.
- ↑ Andersson, M., Structural dynamics of light-driven proton pumps, Structure, 2009, 17(9):1265.
- ↑ Oren, A., Ecology of extremely halophilic microorganisms, Vreeland, R.H., Hochstein, L.I., editors, The Biology of Halophilic Bacteria, CRC Press, Inc., Boca Raton, Florida, 1993, p. 25–54.
- ↑ Walsby, A.E., Gas vesicles, Microbiol Mol Biol Rev, 1994, 58(1):94.
- ↑ Larsen, H., Omang, S., Steensland, H., On the gas vacuoles of the halobacteria, Archiv für Mikrobiologie, 1967, 59(1):197–203.
- ↑ Shammohammadi, H.R., Protective roles of bacterioruberin and intracellular KCl in the resistance of Halobacterium salinarium against DNA-damaging agents, J Radiat Res, 1998, 39(4):251.
- ↑ Ide, H., Takeshi, S., Hiroaki, T., Studies on the antioxidation activity of bacterioruberin, Urakami Found Mem, 1998, 6:127–33.
- ↑ Saito, T., Miyabe, Y., Ide, H., Yamamoto, O., Hydroxyl radical scavenging ability of bacterioruberin, Radiat Phys Chem, 1997, 50(3):267–9.
- ↑ Pfeiffer, F., Schuster, S.C., Broicher, A., Falb, M., Palm, P., Rodewald, K., et al., Evolution in the laboratory: The genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1, Genomics, 2008, 91(4):335–346.
- ↑ Joshia, J.G., Guilda, W.R., Handlera, P., The presence of two species of DNA in some halobacteria, J Mol Biol, 1963, 6(1):34–38.
- ↑ Peck, R.F., DasSarma, S., Krebs, M.P., Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker, Mol Microbiol, 2000, 35(3):667–676.
- ↑ Brijesh, Rajanandam K.S.*, Siva Kiran, R.R., Optimization of hydrogen production by Halobacterium salinarium coupled with E. coli using milk plasma as fermentative substrate, J Biochem Tech, 2011, 3(2):242–244.
- ↑ Reilly, Michael, The Discovery Channel. "World's oldest known DNA discovered". Retrieved 3 September 2010.
- ↑ Park, J. S., Vreeland, R. H., Cho, B. C. , Lowenstein, T. K., Timofeeff, M. N. and Rosenweig, W. D. (December 2009). "Haloarchaeal diversity in 23, 121 and 419 MYA salts". Geobiology 7 (5): 515–23. doi:10.1111/j.1472-4669.2009.00218.x. PMID 19849725.
- ↑ Vreeland, H; Rosenzweig, W D; Lowenstein, T; Satterfield, C; Ventosa, A (December 2006). "Fatty acid and DNA analyses of Permian bacteria isolated from ancient salt crystals reveal differences with their modern relatives". Extremophiles 10 (1): 71–8. doi:10.1007/s00792-005-0474-z. PMID 16133658.
Further reading
- Horia, Todor; Dulmage, Keely; Gillum, Nicholas; Bain, James; Muehlbauer, Michael; Schmid, Amy (2014). "A transcription factor links growth rate and metabolism in the hypersaline adapted archaeon Halobacterium salinarum". Molecular Microbiology 93 (6): 1172–1182. doi:10.1111/mmi.12726.
- Kahaki, Fatemeh Abarghooi; Babaeipour, Valiollah; Memari, Hamid Rajabi; Mofid, Mohammad Reza (2014). "High Overexpression and Purification of Optimized Bacterio-Opsin from Halobacterium Salinarum R1 in E-coli". Applied Biochemistry and Biotechnology 174 (4): 1558–1571. doi:10.1007/s12010-014-1137-2. PMID 25123363.
| ||||||||||||||||||||||||||||||||||