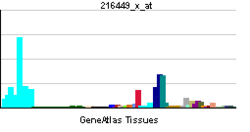
HSP90B1
Heat shock protein 90kDa beta member 1 (HSP90B1), known also as endoplasmin, gp96, grp94 and ERp99, is a chaperone protein that in humans is encoded by the HSP90B1 gene.[1][2]
HSP90B1 is an HSP90 paralogue that is found in the endoplasmic reticulum. It plays critical roles in folding proteins in the secretory pathway such as Toll-like receptors and integrins.[3][4] It has been implicated as an essential immune chaperone to regulate both innate and adaptive immunity.[5] Tumor-derived HSP90B1 (vitespen) has entered clinical trials for cancer immunotherapy.[6][7][8][9]
References
- ↑ Maki RG, Old LJ, Srivastava PK (August 1990). "Human homologue of murine tumor rejection antigen gp96: 5'-regulatory and coding regions and relationship to stress-induced proteins". Proc. Natl. Acad. Sci. U.S.A. 87 (15): 5658–62. doi:10.1073/pnas.87.15.5658. PMC 54386. PMID 2377606.
- ↑ Chen B, Piel WH, Gui L, Bruford E, Monteiro A (December 2005). "The HSP90 family of genes in the human genome: insights into their divergence and evolution". Genomics 86 (6): 627–37. doi:10.1016/j.ygeno.2005.08.012. PMID 16269234.
- ↑ Randow F, Seed B (2001). "Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability.". Nat. Cell Biol. 3 (10): 891–6. doi:10.1038/ncb1001-891. PMID 11584270.
- ↑ Yang Y, et al. (2007). "Heat Shock Protein gp96 Is a Master Chaperone for Toll-like Receptors and Is Important in the Innate Function of Macrophages.". Immunity 26 (2): 215–226. doi:10.1016/j.immuni.2006.12.005. PMC 2847270. PMID 17275357. ,
- ↑ Schild H, Rammensee HG (August 2000). "gp96--the immune system's Swiss army knife". Nat. Immunol. 1 (2): 100–1. doi:10.1038/77770. PMID 11248798.
- ↑ Wood CG, Mulders P (August 2009). "Vitespen: a preclinical and clinical review". Future Oncol 5 (6): 763–74. doi:10.2217/fon.09.46. PMID 19663726.
- ↑ Tosti G, di Pietro A, Ferrucci PF, Testori A (November 2009). "HSPPC-96 vaccine in metastatic melanoma patients: from the state of the art to a possible future". Expert Rev Vaccines 8 (11): 1513–26. doi:10.1586/erv.09.108. PMID 19863242.
- ↑ "NCT00293423". ClinicalTrials.gov, United States National Institutes of Health. Retrieved 2010-04-10.
GP96 Heat Shock Protein-Peptide Complex Vaccine in Treating Patients With Recurrent or Progressive Glioma
- ↑ Bloch, O.; Crane, C. A.; Fuks, Y.; Kaur, R.; Aghi, M. K.; Berger, M. S.; Butowski, N. A.; Chang, S. M.; Clarke, J. L.; McDermott, M. W.; Prados, M. D.; Sloan, A. E.; Bruce, J. N.; Parsa, A. T. (12 December 2013). "Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial". Neuro-Oncology 16: 274–279. doi:10.1093/neuonc/not203.
Further reading
- Srivastava P (2001). "Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses.". Annu Rev Immunol. 20 (1): 395–425. doi:10.1146/annurev.immunol.20.100301.064801. PMID 11861608.
- Li Z, Dai J, Zheng H, et al. (2002). "An integrated view of the roles and mechanisms of heat shock protein gp96-peptide complex in eliciting immune response.". Front. Biosci. 7: d731–51. doi:10.2741/A808. PMID 11861214.
- Dollins DE, et al. (2007). "Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones.". Mol Cell 28 (1): 41–56. doi:10.1016/j.molcel.2007.08.024. PMC 2094010. PMID 17936703.
- Kaul SC, Taira K, Pereira-Smith OM, Wadhwa R (2003). "Mortalin: present and prospective.". Exp. Gerontol. 37 (10-11): 1157–64. doi:10.1016/S0531-5565(02)00135-3. PMID 12470827.
- Schaiff WT, Hruska KA, McCourt DW, et al. (1992). "HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells.". J. Exp. Med. 176 (3): 657–66. doi:10.1084/jem.176.3.657. PMC 2119345. PMID 1512535.
- Zolnierowicz S, Work C, Hutchison K, Fox IH (1990). "Partial separation of platelet and placental adenosine receptors from adenosine A2-like binding protein.". Mol. Pharmacol. 37 (4): 554–9. PMID 2325637.
- Hutchison KA, Nevins B, Perini F, Fox IH (1990). "Soluble and membrane-associated human low-affinity adenosine binding protein (adenotin): properties and homology with mammalian and avian stress proteins.". Biochemistry 29 (21): 5138–44. doi:10.1021/bi00473a020. PMID 2378869.
- Chang SC, Erwin AE, Lee AS (1989). "Glucose-regulated protein (GRP94 and GRP78) genes share common regulatory domains and are coordinately regulated by common trans-acting factors.". Mol. Cell. Biol. 9 (5): 2153–62. PMC 363009. PMID 2546060.
- Anderson SL, Shen T, Lou J, et al. (1994). "The endoplasmic reticular heat shock protein gp96 is transcriptionally upregulated in interferon-treated cells.". J. Exp. Med. 180 (4): 1565–9. doi:10.1084/jem.180.4.1565. PMC 2191700. PMID 7523574.
- Bruneau N, Lombardo D (1995). "Chaperone function of a Grp 94-related protein for folding and transport of the pancreatic bile salt-dependent lipase.". J. Biol. Chem. 270 (22): 13524–33. doi:10.1074/jbc.270.22.13524. PMID 7768954.
- Chavany C, Mimnaugh E, Miller P, et al. (1996). "p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2.". J. Biol. Chem. 271 (9): 4974–7. doi:10.1074/jbc.271.9.4974. PMID 8617772.
- Kuznetsov G, Chen LB, Nigam SK (1997). "Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum.". J. Biol. Chem. 272 (5): 3057–63. doi:10.1074/jbc.272.5.3057. PMID 9006956.
- Hoshino T, Wang J, Devetten MP, et al. (1998). "Molecular chaperone GRP94 binds to the Fanconi anemia group C protein and regulates its intracellular expression.". Blood 91 (11): 4379–86. PMID 9596688.
- Linnik KM, Herscovitz H (1998). "Multiple molecular chaperones interact with apolipoprotein B during its maturation. The network of endoplasmic reticulum-resident chaperones (ERp72, GRP94, calreticulin, and BiP) interacts with apolipoprotein b regardless of its lipidation state.". J. Biol. Chem. 273 (33): 21368–73. doi:10.1074/jbc.273.33.21368. PMID 9694898.
- Delom F, Lejeune PJ, Vinet L, et al. (1999). "Involvement of oxidative reactions and extracellular protein chaperones in the rescue of misassembled thyroglobulin in the follicular lumen.". Biochem. Biophys. Res. Commun. 255 (2): 438–43. doi:10.1006/bbrc.1999.0229. PMID 10049727.
- Reddy RK, Lu J, Lee AS (1999). "The endoplasmic reticulum chaperone glycoprotein GRP94 with Ca(2+)-binding and antiapoptotic properties is a novel proteolytic target of calpain during etoposide-induced apoptosis.". J. Biol. Chem. 274 (40): 28476–83. doi:10.1074/jbc.274.40.28476. PMID 10497210.
- Roher N, Sarno S, Miró F, et al. (2001). "The carboxy-terminal domain of Grp94 binds to protein kinase CK2 alpha but not to CK2 holoenzyme.". FEBS Lett. 505 (1): 42–6. doi:10.1016/S0014-5793(01)02781-8. PMID 11557039.
- Vabulas RM, Braedel S, Hilf N, et al. (2002). "The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway.". J. Biol. Chem. 277 (23): 20847–53. doi:10.1074/jbc.M200425200. PMID 11912201.
- Shin HJ, Kim SS, Cho YH, et al. (2002). "Host cell proteins binding to the encapsidation signal epsilon in hepatitis B virus RNA.". Arch. Virol. 147 (3): 471–91. doi:10.1007/s007050200001. PMID 11958450.
PDB gallery |
|---|
| | 1qy5: Crystal Structure of the N-domain of the ER Hsp90 chaperone GRP94 in complex with the specific ligand NECA |
| 1qy8: Crystal Structure of the N-domain of the ER Hsp90 chaperone GRP94 in complex with Radicicol |
| 1qye: Crystal Structure of the N-domain of the ER Hsp90 chaperone GRP94 in complex with 2-chlorodideoxyadenosine |
| 1u0y: N-Domain Of Grp94, with the Charged Domain, In Complex With the Novel Ligand N-Propyl Carboxyamido Adenosine |
| 1yt2: Crystal Structure of the Unliganded Form of GRP94, the ER Hsp90: Basis for Nucleotide-Induced Conformational Change, GRP94N APO CRYSTAL |
|
|
|