Hydrogen potassium ATPase
| ATPase, H+/K+ exchanging, alpha polypeptide | |
|---|---|
| Identifiers | |
| Symbol | ATP4A |
| Entrez | 495 |
| HUGO | 819 |
| OMIM | 137216 |
| RefSeq | NM_000704 |
| UniProt | P20648 |
| Other data | |
| EC number | 3.6.3.10 |
| Locus | Chr. 19 q13.1 |
| ATPase, H+/K+ exchanging, beta polypeptide | |
|---|---|
| Identifiers | |
| Symbol | ATP4B |
| Entrez | 496 |
| HUGO | 820 |
| OMIM | 137217 |
| RefSeq | NM_000705 |
| UniProt | P51164 |
| Other data | |
| EC number | 3.6.3.10 |
| Locus | Chr. 13 q34 |
Gastric hydrogen potassium ATPase, also known as H+/K+ ATPase, is an enzyme whose purpose is to acidify the stomach.
Biological function and location
The gastric hydrogen potassium ATPase or H+/K+ ATPase is the proton pump of the stomach. It exchanges potassium from the intestinal lumen with cytoplasmic hydronium[1] and is the enzyme primarily responsible for the acidification of the stomach contents and the activation of the digestive enzyme pepsin[2] (see gastric acid).
The H+/K+ ATPase is found in parietal cells, which are highly specialized epithelial cells located in the inner cell lining of the stomach called the gastric mucosa. Parietal cells possess an extensive secretory membrane system and the H+/K+ ATPase is the major protein constituent of these membranes. A small amount of H+/K+ ATPase is also found in the renal medulla.[1]
Genes and protein structure
The H+/K+ ATPase is a heterodimeric protein, the product of 2 genes. The gene ATP4A encodes the H+/K+ ATPase α subunit, and is an ~1000-amino acid protein that contains the catalytic sites of the enzyme and forms the pore through the cell membrane that allows the transport of ions. Hydronium ions bind to two active sites present in the α subunit.[3] The α subunit also has a phosphorylation site (Asp385).[4] The gene ATP4B encodes the β subunit of the H+/K+ ATPase, which is an ~300-amino acid protein with a 36-amino acid N-terminal cytoplasmic domain, a single transmembrane domain, and a highly glycosylated extracellular domain.
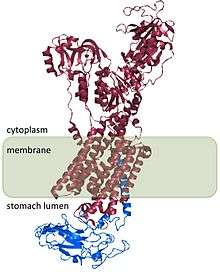
The H+/K+ ATPase β subunit stabilizes the H+/K+ ATPase α subunit and is required for function of the enzyme. The β subunit prevents the pump from running in reverse,[5] and it also appears to contain signals that direct the heterodimer to membrane destinations within the cell, although some of these signals are subordinate to signals found in H+/K+ ATPase α subunit.
The structure of H+/K+ ATPase has been determined for human, dog, hog, rat, and rabbit and is 98% homologous across all species.[1]
Enzyme mechanism and activity
H+/K+ ATPase is a P2-type ATPase, a member of the eukaryotic class of P-type ATPases.[6] Like the Ca2+ and the Na+/K+ ATPases, the H+/K+ ATPase functions as an α, β protomer.[7] Unlike other eukaryotic ATPases, the H+/K+ ATPase is electroneutral, transporting one proton into the stomach lumen per potassium retrieved from the gastric lumen.[6] As an ion pump the H+/K+ ATPase is able to transport ions against a concentration gradient using energy derived from the hydrolysis of ATP. Like all P-type ATPases, a phosphate group is transferred from adenosine triphosphate (ATP) to the H+/K+ ATPase during the transport cycle. This phosphate transfer powers a conformational change in the enzyme that helps drive ion transport.
The hydrogen potassium ATPase is activated indirectly by gastrin that causes ECL cells to release histamine.[8] The histamine binds to H2 receptors on the parietal cell, activating a cAMP-dependent pathway which causes the enzyme to move from the cytoplasmic tubular membranes to deeply folded canaliculi of the stimulated parietal cell.[1] Once localized, the enzyme alternates between two conformations, E1 and E2, to transport ions across the membrane.

The E1 conformation binds a phosphate from ATP and hydronium ion on the cytoplasmic side. The enzyme then changes to the E2 conformation, allowing hydronium to be released in the lumen. The E2 conformation binds potassium, and reverts to the E1 conformation to release phosphate and K+ into the cytoplasm where another ATP can by hydrolyzed to repeat the cycle.[1] The β subunit prevents the E2-P conformation from reverting to the E1-P conformation, making proton pumping unidirectional.[5] The number of ions transported per ATP varies from 2H+/2K+ to 1H+/1K+depending on the pH of the stomach.[10]
Disease relevance and inhibition
Inhibiting the hydrogen potassium pump to decrease stomach acidity has been most common method of treating diseases including gastroesophageal reflux disease (GERD/GORD) and peptic ulcer disease (PUD).[11] Reducing acidity alleviates disease symptoms but does not treat the actual cause of GERD (abnormal relaxation of the esophageal sphincter) or PUD (helicobacter pylori and NSAIDs).[12]
Three drug categories have been used to inhibit H+/K+ ATPases. H2-receptor antagonists, like cimetidine (Tagamet), inhibit the signaling pathway that leads to activation of the ATPase. This type of inhibitor is effective in treating ulcers but does not prevent them from forming, and patients develop tolerance to them is developed after about one week, leading to a 50% reduction in effect.[13] Proton pump inhibitors were later developed, starting with timoprazole in 1975.[13] PPIs are acid-activated prodrugs that inhibit the hydrogen potassium ATPase by binding covalently to active pumps.[14] Current PPIs like omeprazole have a short half-life of 90 minutes.[15] Acid pump antagonists (APAs) or potassium-competitive acid blockers (PCABs) are a third type of inhibitor that blocks acid secretion by binding to the K+ active site.[13] APAs provide faster inhibition than PPIs since they do not require acid activation. Revaprazan was the first APA used clinically in east Asia, and other APAs are being developed since they appear to provide better acid control in clinical trials.[15]
Inactivation of the proton pump can also lead to health problems. A study in mice by Krieg et al.[16] found that a mutation of the pump’s α subunit led to achlorhydria which resulted problems with iron absorption, leading to iron deficiency and anemia. The use of PPIs has not been correlated with an elevated risk of anemia, so the H+/K+ ATPase is thought to aid iron absorption but is not necessarily required.[16]
See also
- Gastric acid
- Parietal cell
- Stomach
- Proton pump inhibitor
- Discovery and Development of Proton Pump Inhibitors
References
- 1 2 3 4 5 Shin, J. M., Munson, K., Vagin, O., & Sachs, G. (2009). "The gastric HK-ATPase: structure, function, and inhibition.". Pflugers Archiv : European Journal of Physiology 457 (3): 609–622. doi:10.1007/s00424-008-0495-4. PMC 3079481. PMID 18536934.
- ↑ Berg, J. M., Tymoczko, J. L., & Stryer, L. (2012). Biochemistry , (7th ed.). New York: W.H. Freeman and Company.
- ↑ Chourasia, M., Sastry, G. M., & Sastry. G. N. (2005). "Proton binding sites and conformational analysis of H+K+-ATPase". Biochemical and Biophysical Research Communications 336 (3): 961–966. doi:10.1016/j.bbrc.2005.08.205.
- ↑ Scheirlinckx, F., Raussens, V., Ruysschaert, J.-M., & Goormaghtigh, E. (2004). "Conformational changes in gastric H+/K+-ATPase monitored by difference Fourier-transform infrared spectroscopy and hydrogen/deuterium exchange". Biochemical Journal (Pt 1 ed.) 382: 121–129. doi:10.1042/BJ20040277.
- 1 2 Abe, K., Tani, K., Nishizawa, T., & Fujiyoshi, Y. (2009). "Inter-subunit interaction of gastric H+,K+-ATPase prevents reverse reaction of the transport cycle". The EMBO Journal 28 (11): 1637–1643. doi:10.1038/emboj.2009.102. PMC 2693145. PMID 19387495.
- 1 2 Shin, J. M. & Sachs, G. (2009). "Ion Motive ATPases: P-type ATPases". eLS. doi:10.1002/9780470015902.a0001379.pub2.
- ↑ Dach, I., Olesen, C., Signor, L., Nissen, P., le Maire, M., Møller, J. V., & Ebel, C. (2012). "Active Detergent-solubilized H+,K+-ATPase Is a Monomer.". The Journal of Biological Chemistry 287 (50, 41963–41978.): 41963–41978. doi:10.1074/jbc.M112.398768.
- ↑ Prinz, C., Kajimura, M., Scott, D., Helander, H., Shin, J., Besancon, M., Bamberg, K., Hersey, S., & Sachs, G. (1992). "Acid secretion and the H,K ATPase of stomach.". The Yale Journal of Biology and Medicine (6, ed.) 65: 577–596.
- ↑ "The gastric HK-ATPase: structure, function, and inhibition.". Pflugers Arch 457: 609–22. Jan 2009. doi:10.1007/s00424-008-0495-4. PMC 3079481. PMID 18536934.
- ↑ Abe, K., Tani, K., Friedrich, T., & Fujiyoshi, Y. (2012). "Cryo-EM structure of gastric H+,K+-ATPase with a single occupied cation-binding site.". Proceedings of the National Academy of Sciences of the United States of America 109 (45): 18401–18406. doi:10.1073/pnas.1212294109.
- ↑ Shin, J. M., Vagin, O., Munson, K., Kidd, M., Modlin, I. M., & Sachs, G. (2008). "Molecular mechanisms in therapy of acid-related diseases.". Cellular and Molecular Life Sciences : CMLS 65 (2): 264–281. doi:10.1007/s00018-007-7249-x.
- ↑ Yeomans, N. D. (2011). "The ulcer sleuths: The search for the cause of peptic ulcers". Journal of Gastroenterology and Hepatology 26: 35–41. doi:10.1111/j.1440-1746.2010.06537.x. PMID 21199512.
- 1 2 3 Sachs, G., Shin, J. M., Vagin, O., Lambrecht, N., Yakubov, I., & Munson, K. (2007). "The Gastric H,K ATPase as a Drug Target: Past, Present, and Future". Journal of Clinical Gastroenterology 41 (Suppl 2): S226–S242. doi:10.1097/MCG.0b013e31803233b7. PMC 2860960. PMID 17575528.
- ↑ Shin J. M., Sachs G. (2008). "Pharmacology of Proton Pump Inhibitors". Current Gastroenterology Reports 10 (6): 528–534. doi:10.1007/s11894-008-0098-4. PMC 2855237. PMID 19006606.
- 1 2 Shin, J. M., & Sachs, G. (2009). "Long lasting inhibitors of the gastric H,K-ATPase". Expert Review of Clinical Pharmacology 2 (5): 461–468. doi:10.1586/ecp.09.33.
- 1 2 Krieg L., Milstein O., Krebs, P., Xia, Y., Beutler B., & Du, X. (2011). "Mutation of the gastric hydrogen-potassium ATPase alpha subunit causes iron-deficiency anemia in mice". Blood 118 (24): 6418–6425. doi:10.1182/blood-2011-04-350082.
External links
- Yao X, Forte JG (2003). "Cell biology of acid secretion by the parietal cell". Annu. Rev. Physiol. 65: 103–31. doi:10.1146/annurev.physiol.65.072302.114200. PMID 12500969.
- Kühlbrandt W (April 2004). "Biology, structure and mechanism of P-type ATPases". Nat. Rev. Mol. Cell Biol. 5 (4): 282–95. doi:10.1038/nrm135410.1038/nrm1354. PMID 15071553.
- Dunbar LA, Caplan MJ (August 2001). "Ion pumps in polarized cells: sorting and regulation of the Na+, K+- and H+, K+-ATPases". J. Biol. Chem. 276 (32): 29617–20. doi:10.1074/jbc.R100023200. PMID 11404365.
- Sachs G, Shin JM, Briving C, Wallmark B, Hersey S (1995). "The pharmacology of the gastric acid pump: the H+,K+ ATPase". Annu. Rev. Pharmacol. Toxicol. 35: 277–305. doi:10.1146/annurev.pa.35.040195.001425. PMID 7598495.
- Potassium Hydrogen ATPase at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||