Gold heptafluoride
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Difluorinegold(V) fluoride | |||
| Other names
Gold heptafluoride | |||
| Properties | |||
| AuF7 | |||
| Molar mass | 322.956 g/mol | ||
| Hazards | |||
| Main hazards | Corrosive, toxic | ||
| Related compounds | |||
| Other cations |
ReF7, IF7 | ||
| Related compounds |
AuF3, AuF5 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
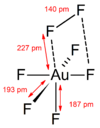
Gold heptafluoride is a gold(V) compound with the empirical formula AuF7. The synthesis of this compound was first reported in 1986.[1] Current calculations suggest that it may be the first difluorine complex, AuF5·F2. The gold(V) difluorine complex is calculated to be 205 kJ/mol more stable than gold(VII) fluoride. The vibrational frequency at 734 cm−1 is the hallmark of the end-on coordinated difluorine molecule.[2]
References
- ↑ Timakov, A. A.; Prusakov, V. N.; Drobyshevskii, Y. V. (1986). Dokl. Akad. Nauk SSSR (in Russian) 291: 125–128. Missing or empty
|title=(help) - ↑ Himmel, Daniel; Riedel, Sebastian (2007-05-31). "After 20 Years, Theoretical Evidence That "AuF7" Is Actually AuF5·F2". Inorganic Chemistry 46 (13): 5338–5342. doi:10.1021/ic700431s. Cite uses deprecated parameter
|coauthors=(help)
| ||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the Monday, November 16, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.