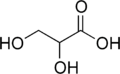
Glyceric acid
 | |
| Names | |
|---|---|
| IUPAC name
2,3-Dihydroxypropanoic acid | |
| Other names
Glycerate | |
| Identifiers | |
| 473-81-4 | |
| ChemSpider | 732 |
| Jmol interactive 3D | Image |
| PubChem | 752 |
| |
| |
| Properties | |
| C3H6O4 | |
| Molar mass | 106.08 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Glyceric acid is a natural three-carbon sugar acid. Salts and esters of glyceric acid are known as glycerates.
glycerol after reacting with gym-an oxygen giving reagent (such as KMnO4+H2SO4) produced glyceric acid.
KMnO4+3H2SO4=K2SO4+3H20+5[O] CH2OH-CHOH-CH2OH+[O]=CH2OH-CHOH-COOH+H2O
Biochemistry
Several phosphate derivatives of glyceric acid, including 2-phosphoglyceric acid, 3-phosphoglyceric acid, 2,3-bisphosphoglyceric acid, and 1,3-bisphosphoglyceric acid, are important biochemical intermediates in Glycolysis.
References
- ↑ Merck Index, 11th Edition, 4378.
This article is issued from Wikipedia - version of the Wednesday, July 08, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.