Interferon gamma
| Interferon gamma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystal structure of a biologically active single chain mutant of human interferon gamma | |||||||||
| Identifiers | |||||||||
| Symbol | IFN gamma | ||||||||
| Pfam | PF00714 | ||||||||
| Pfam clan | CL0053 | ||||||||
| InterPro | IPR002069 | ||||||||
| SCOP | 1rfb | ||||||||
| SUPERFAMILY | 1rfb | ||||||||
| |||||||||
| Systematic (IUPAC) name | |
|---|---|
|
Human interferon gamma-1b | |
| Clinical data | |
| Trade names | Actimmune |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a601152 |
| Identifiers | |
| CAS Number |
82115-62-6 |
| ATC code | L03AB03 |
| DrugBank |
DB00033 |
| ChemSpider | none |
| ChEMBL |
CHEMBL1201564 |
| Chemical data | |
| Formula | C761H1206N214O225S6 |
| Molar mass | 17145.6 g/mol |
| | |
Interferon gamma (IFNγ) is a dimerized soluble cytokine that is the only member of the type II class of interferons.[2] The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock as a product of human leukocytes stimulated with phytohemagglutinin, and by others as a product of antigen-stimulated lymphocytes[3][4] or tuberculin-sensitized mouse peritoneal lymphocytes[5] challenged with PPD; the resulting supernatants were shown to inhibit growth of vesicular stomatitis virus. Those reports also contained the basic observation underlying the now widely employed interferon gamma release assay used to test for tuberculosis. In humans, the IFNγ protein is encoded by the IFNG gene.[6][7]
Function
IFNγ, or type II interferon, is a cytokine that is critical for innate and adaptive immunity against viral, some bacterial and protozoal infections. IFNγ is an important activator of macrophages and inducer of Class II major histocompatibility complex (MHC) molecule expression. Aberrant IFNγ expression is associated with a number of autoinflammatory and autoimmune diseases. The importance of IFNγ in the immune system stems in part from its ability to inhibit viral replication directly, and most importantly from its immunostimulatory and immunomodulatory effects. IFNγ is produced predominantly by natural killer (NK) and natural killer T (NKT) cells as part of the innate immune response, and by CD4 Th1 and CD8 cytotoxic T lymphocyte (CTL) effector T cells once antigen-specific immunity develops.[8][9]
Structure
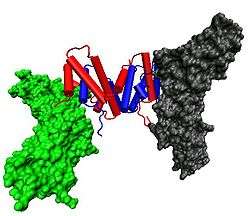
The IFNγ monomer consists of a core of six α-helices and an extended unfolded sequence in the C-terminal region.[10][1] This is shown in the structural models below. The α-helices in the core of the structure are numbered 1 to 6.

The biologically active dimer is formed by anti-parallel inter-locking of the two monomers as shown below. In the cartoon model, one monomer is shown in red, the other in blue.

Receptor binding
Cellular responses to IFNγ are activated through its interaction with a heterodimeric receptor consisting of Interferon gamma receptor 1 (IFNGR1) and Interferon gamma receptor 2 (IFNGR2). IFNγ binding to the receptor activates the JAK-STAT pathway. IFNγ also binds to the glycosaminoglycan heparan sulfate (HS) at the cell surface. However, in contrast to many other heparan sulfate binding proteins, where binding promotes biological activity, the binding of IFNγ to HS inhibits its biological activity.[11]
The structural models shown in figures 1-3 for IFNγ[1] are all shortened at their C-termini by 17 amino acids. Full length IFNγ is 143 amino acids long, the models are 126 amino acids long. Affinity for heparan sulfate resides solely within the deleted sequence of 17 amino acids.[12] Within this sequence of 17 amino acids lie two clusters of basic amino acids termed D1 and D2, respectively. Heparan sulfate interacts with both of these clusters.[13] In the absence of heparan sulfate the presence of the D1 sequence increases the rate at which IFNγ-receptor complexes form.[11] Interactions between the D1 cluster of amino acids and the receptor may be the first step in complex formation. By binding to D1 HS may compete with the receptor and prevent active receptor complexes from forming.
The biological significance of heparan sulfates interaction with IFNγ is unclear, however binding of the D1 cluster to HS may protect it from proteolytic cleavage.[13]
Biological activity
IFNγ is secreted by T helper cells (specifically, Th1 cells), cytotoxic T cells (TC cells) and NK cells only. IFNγ is the only Type II interferon and it is serologically distinct from Type I interferons; it is acid-labile, while the type I variants are acid-stable.
IFNγ has antiviral, immunoregulatory, and anti-tumor properties.[14] It alters transcription in up to 30 genes producing a variety of physiological and cellular responses. Among the effects are:
- Promotes NK cell activity
- Increase antigen presentation and lysosome activity of macrophages.
- Activate inducible Nitric Oxide Synthase iNOS
- Induces the production of IgG2a and IgG3 from activated plasma B cells
- Promotes Th1 differentiation by upregulating the transcription factor T-bet, ultimately leading to cellular immunity: cytotoxic CD8+ T-cells and macrophage activity - while suppressing Th2 differentiation, which would cause a humoral (antibody) response
- Cause normal cells to increase expression of class I MHC molecules as well as class II MHC on antigen-presenting cells—to be specific, through induction of antigen processing genes, including subunits of the immunoproteasome (MECL1, LMP2, LMP7), as well as TAP and ERAAP in addition possibly to the direct upregulation of MHC heavy chains and B2-microglobulin itself
- Promotes adhesion and binding required for leukocyte migration
- Induces the expression of intrinsic defense factors—for example, with respect to retroviruses, relevant genes include TRIM5alpha, APOBEC, and Tetherin, representing directly antiviral effects
IFNγ is the primary cytokine that defines Th1 cells: Th1 cells secrete IFNγ, which in turn causes more undifferentiated CD4+ cells (Th0 cells) to differentiate into Th1 cells, representing a positive feedback loop—while suppressing Th2 cell differentiation. (Equivalent defining cytokines for other cells include IL-4 for Th2 cells and IL-17 for Th17 cells.)
NK cells and CD8+ cytotoxic T cells also produce IFNγ. IFNγ suppresses osteoclast formation by rapidly degrading the RANK adaptor protein TRAF6 in the RANK-RANKL signaling pathway, which otherwise stimulates the production of NF-κB.
Activity in granuloma formation
A granuloma is the body's way of dealing with a substance it cannot remove or sterilize. Infectious causes of granulomas (infections are typically the most common cause of granulomas) include tuberculosis, leprosy, histoplasmosis, cryptococcosis, coccidioidomycosis, blastomycosis, and cat scratch disease. Examples of non-infectious granulomatous diseases are sarcoidosis, Crohn's disease, berylliosis, giant-cell arteritis, granulomatosis with polyangiitis, Churg–Strauss syndrome, pulmonary rheumatoid nodules, and aspiration of food and other particulate material into the lung. The infectious pathophysiology of granulomas is discussed primarily here.
The key association between IFNγ and granulomas is that IFNγ activates macrophages so that they become more powerful in killing intracellular organisms. Activation of macrophages by IFNγ from Th1 helper cells in mycobacterial infections allows the macrophages to overcome the inhibition of phagolysosome maturation caused by mycobacteria (to stay alive inside macrophages).[15] The first steps in INFγ-induced granuloma formation are activation of Th1 helper cells by macrophages releasing IL-1 and IL-12 in the presence of intracellular pathogens, and presentation of antigens from those pathogens. Next the Th1 helper cells aggregate around the macrophages and release IFNγ, which activates the macrophages. Further activation of macrophages causes a cycle of further killing of intracellular bacteria, and further presentation of antigens to Th1 helper cells with further release of IFNγ. Finally, macrophages surround the Th1 helper cells and become fibroblast-like cells walling off the infection.
Activity during pregnancy
Uterine Natural Killer cells (NK) secrete high levels of chemoattractants, such as IFNγ. IFNγ dilates and thins the walls of maternal spiral arteries to enhance blood flow to the implantation site. This remodeling aids in the development of the placenta as it invades the uterus in its quest for nutrients. IFNγ knockout mice fail to initiate normal pregnancy-induced modification of decidual arteries. These models display abnormally low amounts of cells or necrosis of decidua.[16]
Therapeutic use
Interferon-γ 1b is approved by the U.S. Food and Drug Administration to treat chronic granulomatous disease[17] and osteopetrosis.[18]
It was not approved to treat idiopathic pulmonary fibrosis (IPF). In 2002, the manufacturer InterMune issued a press release saying that phase III data demonstrated survival benefit in IPF and reduced mortality by 70% in patients with mild to moderate disease. The U.S. Department of Justice charged that the release contained false and misleading statements. InterMune's chief executive, Scott Harkonen, was accused of manipulating the trial data, was convicted in 2009 of wire fraud, and was sentenced to fines and community service. Harkonen appealed his conviction to the U.S. Court of Appeals for the Ninth Circuit, and lost.[19]
It is being studied at the Children’s Hospital of Philadelphia for the treatment of Friedreich's ataxia.[20]
Although not officially approved, Interferon-γ has also been shown to be effective in treating patients with moderate to severe atopic dermatitis. [21] [22] [23]
It is manufactured by InterMune as Actimmune and costs around USD300 per vial.
Interactions
Interferon-γ has been shown to interact with Interferon gamma receptor 1.[24][25]
Regulation
There is evidence that interferon-gamma expression is regulated by a pseudoknotted element in its 5' UTR.[26] There is also evidence that interferon-gamma is regulated either directly or indirectly by the microRNAs: miR-29.[27] Furthermore, there is evidence that interferon-gamma expression is regulated via GAPDH in T-cells. This interaction takes place in the 3'UTR, where binding of GAPDH prevents the translation of the mRNA sequence.[28]
References
- 1 2 3 4 5 6 PDB: 1FG9; Thiel DJ, le Du MH, Walter RL, D'Arcy A, Chène C, Fountoulakis M, Garotta G, Winkler FK, Ealick SE (September 2000). "Observation of an unexpected third receptor molecule in the crystal structure of human interferon-gamma receptor complex". Structure 8 (9): 927–36. doi:10.1016/S0969-2126(00)00184-2. PMID 10986460.
- ↑ Gray PW, Goeddel DV (August 1982). "Structure of the human immune interferon gene". Nature 298 (5877): 859–63. doi:10.1038/298859a0. PMID 6180322.
- ↑ Wheelock, EF, Interferon-like virus inhibitor induced in human leukocytes by phytohemagglutinin. Science 149, 310-311, 1965. It was also shown to be produced in human lymphocytes
- ↑ Green JA, Cooperband SR, Kibrick S (1969). "Immune specific induction of interferon production in cultures of human blood lymphocytes". Science 164 (3886): 1415–1417. doi:10.1126/science.164.3886.1415. PMID 5783715.
- ↑ Milstone LM, Waksman BH (1970). "Release of virus inhibitor from tuberculin-sensitized peritoneal cells stimulated by antigen". J Immunol 105 (5): 1068–1071. PMID 4321289.
- ↑ Naylor SL, Sakaguchi AY, Shows TB, Law ML, Goeddel DV, Gray PW (March 1983). "Human immune interferon gene is located on chromosome 12". J. Exp. Med. 157 (3): 1020–7. doi:10.1084/jem.157..1020. PMC 2186972. PMID 6403645.
- ↑ "Entrez Gene: IFNGR2".
- ↑ "Entrez Gene: INFG".
- ↑ Schoenborn JR, Wilson CB (2007). "Regulation of interferon-gamma during innate and adaptive immune responses". Adv. Immunol. 96: 41–101. doi:10.1016/S0065-2776(07)96002-2. PMID 17981204.
- ↑ Ealick SE, Cook WJ, Vijay-Kumar S, Carson M, Nagabhushan TL, Trotta PP, Bugg CE (May 1991). "Three-dimensional structure of recombinant human interferon-gamma". Science 252 (5006): 698–702. doi:10.1126/science.1902591. PMID 1902591.
- 1 2 Sadir R, Forest E, Lortat-Jacob H (May 1998). "The heparan sulfate binding sequence of interferon-gamma increased the on rate of the interferon-gamma-interferon-gamma receptor complex formation". J. Biol. Chem. 273 (18): 10919–10925. doi:10.1074/jbc.273.18.10919. PMID 9556569.
- ↑ Vanhaverbeke C, Simorre JP, Sadir R, Gans P, Lortat-Jacob H (November 2004). "NMR characterization of the interaction between the C-terminal domain of interferon-gamma and heparin-derived oligosaccharides". Biochem. J. 384 (Pt 1): 93–9. doi:10.1042/BJ20040757. PMC 1134092. PMID 15270718.
- 1 2 Lortat-Jacob H, Grimaud JA (March 1991). "Interferon-gamma binds to heparan sulfate by a cluster of amino acids located in the C-terminal part of the molecule". FEBS Lett. 280 (1): 152–154. doi:10.1016/0014-5793(91)80225-R. PMID 1901275.
- ↑ Schroder K, Hertzog PJ, Ravasi T, Hume DA (February 2004). "Interferon-gamma: an overview of signals, mechanisms and functions". J. Leukoc. Biol. 75 (2): 163–89. doi:10.1189/jlb.0603252. PMID 14525967.
- ↑ Citations needed
- ↑ Ashkar AA, Di Santo JP, Croy BA (July 2000). "Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy". J. Exp. Med. 192 (2): 259–70. doi:10.1084/jem.192.2.259. PMC 2193246. PMID 10899912.
- ↑ Todd PA, Goa KL (January 1992). "Interferon gamma-1b. A review of its pharmacology and therapeutic potential in chronic granulomatous disease". Drugs 43 (1): 111–22. doi:10.2165/00003495-199243010-00008. PMID 1372855.
- ↑ Key LL, Ries WL, Rodriguiz RM, Hatcher HC (July 1992). "Recombinant human interferon gamma therapy for osteopetrosis". J. Pediatr. 121 (1): 119–24. doi:10.1016/S0022-3476(05)82557-0. PMID 1320672.
- ↑ Silverman E (September 2013). "The line between scientific uncertainty and promotion of snake oil". BMJ 347: f5687–f5687. doi:10.1136/bmj.f5687. PMID 24055923.
- ↑ "Open-label pilot study of interferon gamma-1b (Actimmune™) for the treatment of Friedreich Ataxia" (PDF). FARA. 10 June 2013. Retrieved 4 October 2013.
- ↑ Akhavan A, Rudikoff D (2008). "Atopic Dermatitis: Systemic Immunosuppressive Therapy" (PDF). Seminars in Cutaneous Medicine and Surgery 27 (2): 151–155. doi:10.1016/j.sder.2008.04.004. PMID 18620137. Retrieved 16 October 2014.
- ↑ Schneider LC, Baz Z, Zarcone C, Zurakowski D (1998). "Long-Term Therapy with Recombinant Interferon-Gamma (rIFN-γ) for Atopic Dermatitis". Annals of Allergy, Asthma and Immunology 80 (3): 263–268. doi:10.1016/S1081-1206(10)62968-7. PMID 9532976. Retrieved 16 October 2014.
- ↑ Hanifin JM, Schneider LC, Leung DY, Ellis CN, Jaffe HS, Izu AE, Bucalo LR, Hirabayashi SE, Tofte SJ, Cantu-Gonzales G (1993). "Recombinant interferon gamma therapy for atopic dermatitis.". Journal of the American Academy of Dermatology 28 (2 Pt 1): 189–97. doi:10.1016/0190-9622(93)70026-p. PMID 8432915.
- ↑ Thiel DJ, le Du MH, Walter RL, D'Arcy A, Chène C, Fountoulakis M, Garotta G, Winkler FK, Ealick SE (September 2000). "Observation of an unexpected third receptor molecule in the crystal structure of human interferon-gamma receptor complex". Structure 8 (9): 927–36. doi:10.1016/S0969-2126(00)00184-2. PMID 10986460.
- ↑ Kotenko SV, Izotova LS, Pollack BP, Mariano TM, Donnelly RJ, Muthukumaran G, Cook JR, Garotta G, Silvennoinen O, Ihle JN (September 1995). "Interaction between the components of the interferon gamma receptor complex". J. Biol. Chem. 270 (36): 20915–21. doi:10.1074/jbc.270.36.20915. PMID 7673114.
- ↑ Ben-Asouli Y, Banai Y, Pel-Or Y, Shir A, Kaempfer R (2002). "Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR". Cell 108 (2): 221–232. doi:10.1016/S0092-8674(02)00616-5. PMID 11832212.
- ↑ Asirvatham AJ, Gregorie CJ, Hu Z, Magner WJ, Tomasi TB (2008). "MicroRNA targets in immune genes and the Dicer/Argonaute and ARE machinery components.". Mol Immunol 45 (7): 1995–2006. doi:10.1016/j.molimm.2007.10.035. PMC 2678893. PMID 18061676.
- ↑ Chang CH, Curtis JD, Maggi LB, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL (2013). "Posttranscriptional control of T cell effector function by aerobic glycolysis". Cell 153 (6): 1239–51. doi:10.1016/j.cell.2013.05.016. PMC 3804311. PMID 23746840.
Further reading
- Hall, Stephen K. (1997). A commotion in the blood: life, death, and the immune system. New York: Henry Holt. ISBN 0-8050-5841-9.
- Ikeda H, Old LJ, Schreiber RD (2002). "The roles of IFN gamma in protection against tumor development and cancer immunoediting.". Cytokine Growth Factor Rev. 13 (2): 95–109. doi:10.1016/S1359-6101(01)00038-7. PMID 11900986.
- Chesler DA, Reiss CS (2003). "The role of IFN-gamma in immune responses to viral infections of the central nervous system.". Cytokine Growth Factor Rev. 13 (6): 441–54. doi:10.1016/S1359-6101(02)00044-8. PMID 12401479.
- Dessein A, Kouriba B, Eboumbou C, Dessein H, Argiro L, Marquet S, Elwali NE, Rodrigues V, Li Y, Doumbo O, Chevillard C (2005). "Interleukin-13 in the skin and interferon-gamma in the liver are key players in immune protection in human schistosomiasis.". Immunol. Rev. 201: 180–90. doi:10.1111/j.0105-2896.2004.00195.x. PMID 15361241.
- Joseph AM, Kumar M, Mitra D (2005). "Nef: "necessary and enforcing factor" in HIV infection.". Curr. HIV Res. 3 (1): 87–94. doi:10.2174/1570162052773013. PMID 15638726.
- Copeland KF (2006). "Modulation of HIV-1 transcription by cytokines and chemokines.". Mini reviews in medicinal chemistry 5 (12): 1093–101. doi:10.2174/138955705774933383. PMID 16375755.
- Chiba H, Kojima T, Osanai M, Sawada N (2006). "The significance of interferon-gamma-triggered internalization of tight-junction proteins in inflammatory bowel disease.". Sci. STKE 2006 (316): pe1. doi:10.1126/stke.3162006pe1. PMID 16391178.
- Tellides G, Pober JS (2007). "Interferon-gamma axis in graft arteriosclerosis.". Circ. Res. 100 (5): 622–32. doi:10.1161/01.RES.0000258861.72279.29. PMID 17363708.
| |||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article incorporates text from the United States National Library of Medicine, which is in the public domain.