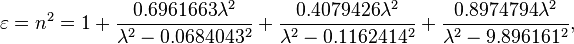Fused quartz

Fused quartz or fused silica is glass consisting of silica in amorphous (non-crystalline) form. It differs from traditional glasses in containing no other ingredients, which are typically added to glass to lower the melt temperature. Fused silica, therefore, has high working and melting temperatures. The optical and thermal properties of fused quartz are superior to those of other types of glass due to its purity. For these reasons, it finds use in situations such as semiconductor fabrication and laboratory equipment. It has better ultraviolet transmission than most other glasses, and so is used to make lenses and other optics for the ultraviolet spectrum. Its low coefficient of thermal expansion also makes it a useful material for precision mirror substrates.[1]
Production
Feedstock
Fused quartz is produced by fusing (melting) high-purity silica sand, which consists of quartz crystals. Quartz contains only silicon and oxygen, although commercial quartz glass often contains impurities. The most dominant impurities are aluminium and titanium.[2]
Fusion
Melting is effected at approximately 2000 °C using either an electrically heated furnace (electrically fused) or a gas/oxygen-fuelled furnace (flame fused). Fused silica can be made from almost any silicon-rich chemical precursor, usually using a continuous process which involves flame oxidation of volatile silicon compounds to silicon dioxide, and thermal fusion of the resulting dust (although there are alternative processes). This results in a transparent glass with an ultra-high purity and improved optical transmission in the deep ultraviolet. One common method involves adding silicon tetrachloride to a hydrogen–oxygen flame, however use of this precursor results in environmentally unfriendly by-products including chlorine and hydrochloric acid. To eliminate these by-products, new processes have been developed using an alternative feedstock, which has also resulted in a higher purity fused silica with further improved deep ultraviolet transmission.
Product quality
Fused quartz is normally transparent, the process of fusion results in a material that is translucent. The material can however appear opaque owing to the presence small air bubbles trapped within the material. The water content (and therefore infrared transmission of fused quartz and fused silica) is determined by the manufacturing process. Flame fused material always has a higher water content due to the combination of the hydrocarbons and oxygen fuelling the furnace forming hydroxyl [OH] groups within the material. An IR grade material typically has an [OH] content of <10 parts per million.
Applications
Most of the applications of fused silica exploit its wide transparency range, which extends from the UV to the near IR. Fused silica is the key starting material for optical fiber, used for telecommunications.
Because of its strength and high melting point (compared to ordinary glass), fused silica is used as an envelope for halogen lamps and high-intensity discharge lamps, which must operate at a high envelope temperature to achieve their combination of high brightness and long life. Vacuum tubes with silica envelopes allowed for radiation cooling by incandescent anodes.
Because of its strength fused silica was used in deep diving vessels such as the Bathysphere and Benthoscope.
The combination of strength, thermal stability, and UV transparency makes it an excellent substrate for projection masks for photolithography.
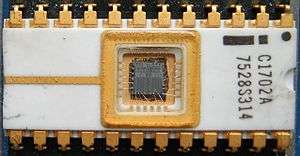
Its UV transparency also finds uses in the semiconductor industry; an EPROM, or erasable programmable read only memory, is a type of memory chip that retains its data when its power supply is switched off, but which can be erased by exposure to strong ultraviolet light. EPROMs are recognizable by the transparent fused quartz window which sits on top of the package, through which the silicon chip is visible, and which permits exposure to UV light during erasing.
Due to the thermal stability and composition it is used in semiconductor fabrication furnaces.
Fused quartz has nearly ideal properties for fabricating first surface mirrors such as those used in telescopes. The material behaves in a predictable way and allows the optical fabricator to put a very smooth polish onto the surface and produce the desired figure with fewer testing iterations. In some instances, a high-purity UV grade of fused quartz has been used to make several of the individual uncoated lens elements of special purpose lenses including the Zeiss 105mm f/4.3 UV Sonnar, a lens formerly made for the Hasselblad camera, and the Nikon UV-Nikkor 105mm f/4.5 (presently sold as the Nikon PF10545MF-UV) lens. These lenses are used for UV photography, as the quartz glass has a lower extinction rate than lens made with more common flint or crown glass formulas.
Fused quartz is also the material used for modern glass instruments such as the glass harp and the verrophone, and is also used for new builds of the historical glass harmonica. Here, the superior strength and structure of fused quartz gives it a greater dynamic range than the historically used lead crystal, and a clearer sound.
Refractory material applications
Fused silica as an industrial raw material is used to make various refractory shapes such as crucibles, trays, shrouds, and rollers for many high-temperature thermal processes including steelmaking, investment casting, and glass manufacture. Refractory shapes made from fused silica have excellent thermal shock resistance and are chemically inert to most elements and compounds including virtually all acids, regardless of concentration, except hydrofluoric acid which is very reactive even in fairly low concentrations. Translucent fused silica tubes are commonly used to sheathe electric elements in room heaters, industrial furnaces and other similar applications.
Owing to its low mechanical damping at ordinary temperatures, it is used for high-Q resonators, in particular, for wine-glass resonator of hemispherical resonator gyro (HRG).[3][4]
Quartz glassware is occasionally used in chemistry laboratories when standard borosilicate glass cannot withstand high temperatures; it is more commonly found as a very basic element, such as a tube in a furnace, or as a flask, the elements in direct exposure to the heat.
Physical properties
The extremely low coefficient of thermal expansion, about 5.5×10−7/°C (20–320 °C), accounts for its remarkable ability to undergo large, rapid temperature changes without cracking (see thermal shock).

Fused quartz is prone to phosphorescence and "solarisation" (purplish discoloration) under intense UV illumination, as is often seen in flashtubes. "UV grade" synthetic fused silica (sold under various tradenames including "HPFS", "Spectrosil" and "Suprasil") has a very low metallic impurity content making it transparent deeper into the ultraviolet. An optic with a thickness of 1 cm will have a transmittance of about 50% at a wavelength of 170 nm, which drops to only a few percent at 160 nm. However, its infrared transmission is limited by strong water absorptions at 2.2 μm and 2.7 μm.
"Infrared grade" fused quartz (tradenames "Infrasil", "Vitreosil IR" and others) which is electrically fused, has a greater presence of metallic impurities, limiting its UV transmittance wavelength to around 250 nm, but a much lower water content, leading to excellent infrared transmission up to 3.6 μm wavelength. All grades of transparent fused quartz/fused silica have nearly identical physical properties.

Optical properties
The optical dispersion of fused silica can be approximated by the following Sellmeier equation:[5]
where the wavelength  is measured in micrometers. This equation is valid between 0.21 and 3.71 micrometers and at 20 °C.[5] Its validity was confirmed for wavelengths up to 6.7 µm.[6] Experimental data for the real (refractive index) and imaginary (absorption index) parts of the complex refractive index of fused quartz reported in the literature over the spectral range from 30 nm to 1000 µm has been reviewed by Kitamura et al.[6] and are available online.
is measured in micrometers. This equation is valid between 0.21 and 3.71 micrometers and at 20 °C.[5] Its validity was confirmed for wavelengths up to 6.7 µm.[6] Experimental data for the real (refractive index) and imaginary (absorption index) parts of the complex refractive index of fused quartz reported in the literature over the spectral range from 30 nm to 1000 µm has been reviewed by Kitamura et al.[6] and are available online.
Typical properties of clear fused silica
- Density: 2.203 g/cm3
- Hardness: 5.3–6.5 (Mohs scale), 8.8 GPa
- Tensile strength: 48.3 MPa
- Compressive strength: >1.1 GPa
- Bulk modulus: ~37 GPa
- Rigidity modulus: 31 GPa
- Young's modulus: 71.7 GPa
- Poisson's ratio: 0.17
- Lamé elastic constants: λ=15.87 GPa, μ=31.26 GPa
- Coefficient of thermal expansion: 5.5×10−7/°C (average from 20 °C to 320 °C)
- Thermal conductivity: 1.3 W/(m·K)
- Specific heat capacity: 45.3 J/(mol·K)
- Softening point: c. 1665 °C
- Annealing point: c. 1140 °C
- Strain point: 1070 °C
- Electrical resistivity: >1018 Ω·m
- Dielectric constant: 3.75 at 20 °C 1 MHz
- Dielectric loss factor: less than 0.0004 at 20 °C 1 MHz
- Index of refraction: at 587.6 nm (nd): 1.4585
- Change of refractive index with temperature (0 to 700 °C): 1.28×10−5/°C (between 20 and 30 °C)[5]
- Strain-optic coefficients: p11=0.113, p12=0.252.
- Hamaker constant: A=6.5×10−20 J.
- Dielectric strength: 250–400 kV/cm at 20 °C[7]
- Surface Tension: .300 N/m at 1800 - 2400 °C[8]
See also
References
- ↑ De Jong, Bernard H. W. S.; Beerkens, Ruud G. C.; Van Nijnatten, Peter A. (2000). "Glass". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a12_365. ISBN 3-527-30673-0.
- ↑ Chemical purity of fused quartz / fused silica, www.heraeus-quarzglas.com
- ↑ An Overview of MEMS Inertial Sensing Technology, February 1, 2003
- ↑ Penn, Steven D.; Harry, Gregory M.; Gretarsson, Andri M.; Kittelberger, Scott E.; Saulson, Peter R.; Schiller, John J.; Smith, Joshua R.; Swords, Sol O. (2001). "High quality factor measured in fused silica". Review of Scientific Instruments 72 (9): 3670. doi:10.1063/1.1394183.
- 1 2 3 Malitson, I. H. (October 1965). "Interspecimen Comparison of the Refractive Index of Fused Silica" (PDF). Journal of the Optical Society of America 55 (10): 1205–1209. doi:10.1364/JOSA.55.001205. Retrieved 2014-07-12.
- 1 2 Kitamura, Rei; Pilon, Laurent; Jonasz, Miroslaw (2007-11-19). "Optical Constants of Silica Glass From Extreme Ultraviolet to Far Infrared at Near Room Temperatures" (PDF). Applied Optics 46 (33): 8118–8133. doi:10.1364/AO.46.008118. Retrieved 2014-07-12.
- ↑ Fused silica datapage
- ↑ Surface tension and viscosity measurement of optical glasses using a scanning CO2 laser
External links
- "Frozen Eye to Bring New Worlds into View" Popular Mechanics, June 1931 General Electrics, West Lynn Massachusetts Labs work on large fuzed quartz blocks
| ||||||||||||||||||||||||||||||