Furazolidone
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
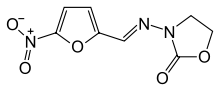
3-{[(5-nitro-2-furyl)methylene]amino}-1,3-oxazolidin-2-one | |
| Clinical data | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral-Local |
| Identifiers | |
| CAS Number |
67-45-8 |
| ATC code | G01AX06 QJ01XE90 |
| PubChem | CID 3435 |
| DrugBank |
DB00614 |
| ChemSpider |
3317 |
| UNII |
5J9CPU3RE0 |
| KEGG |
C07999 |
| ChEMBL |
CHEMBL1103 |
| Chemical data | |
| Formula | C8H7N3O5 |
| Molar mass | 225.16 |
| |
| |
| | |
Furazolidone is a nitrofuran antibacterial agent. It is marketed by Roberts Laboratories under the brand name Furoxone and by GlaxoSmithKline as Dependal-M. Diafuron, medaron
Uses
Furazolidone has been used in human and veterinary medicine. It has a broad spectrum of activity being active against
- Gram positive
- Gram negative
- Escherichia coli
- Salmonella dublin
- Salmonella typhimurium
- Protozoa
Use in humans
In humans it has been used to treat diarrhoea and enteritis caused by bacteria or protozoan infections. It has been used to treat traveler's diarrhoea, cholera and bacteremic salmonellosis. Use in treating Helicobacter pylori infections has also been proposed.[1]
Furazolidone is also used for giardiasis (due to Giardia lamblia), though it is not a first line treatment.[2]
Use in animals
As a veterinary medicine, furazolidone has been used with some success to treat salmonids for Myxobolus cerebralis infections.
It has also been used in aquaculture.[3]
Since furazolidone is a nitrofuran antibiotic, its use in food animals is currently prohibited by the FDA under the Animal Medicinal Drug Use Clarification Act, 1994.[4]
Use in laboratory
It is used to differentiate micrococci and staphylococci.
Mechanism
It is believed to work by crosslinking of DNA.[5]
Side effects
Furazolidone is no longer available in the US. Though an effective antibiotic when all others fail, against extremely drug resistant infections, it has many side effects, and as with other nitrofurans generally, minimum inhibitory concentrations also produce systemic toxicity (tremors, convulsions, peripheral neuritis, gastrointestinal disturbances, depression of spermatogenesis.) Nitrofurans are recognized by FDA as mutagens/carcinogens, and can no longer be used since 1991.[6]
See also
References
- ↑ Machado RS, Silva MR, Viriato A (2008). "Furazolidone, tetracycline and omeprazole: a low-cost alternative for Helicobacter pylori eradication in children". Jornal de pediatria 84 (2): 160–5. doi:10.2223/JPED.1772. PMID 18372934.
- ↑ Petri WA (February 2005). "Treatment of Giardiasis". Curr Treat Options Gastroenterol 8 (1): 13–17. doi:10.1007/s11938-005-0047-3. PMID 15625030.
- ↑ Meng J, Mangat SS, Grudzinski IP, Law FC (1998). "Evidence of 14C-furazolidone metabolite binding to the hepatic DNA of trout". Drug Metabol Drug Interact 14 (4): 209–19. PMID 10694929.
- ↑ Bagley, Clell. "Drugs Prohibited from Extralabel Use in Animals". Retrieved 14 April 2014.
- ↑ "DrugBank: Showing Furazolidone (DB00614)". Retrieved 2008-12-19.
- ↑ http://caraga.da.gov.ph/services/banmed-Nitrofurans.htm Archived September 24, 2007 at the Wayback Machine
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||