Carbonation
See also Carbonatation (calcium carbonate), Carbonization (charcoal), Carburizing (adding carbon to metal), or Fizz (disambiguation).

Bubbles of carbon dioxide float to the surface of a carbonated soft drink.
Carbonation can refer to two chemical processes involving carbon dioxide:
- The dissolution in a liquid, also known as fizz.
- The incorporation into chemical compounds.
Dissolution in a liquid
The process usually involves carbon dioxide under high pressure. When the pressure is reduced, the carbon dioxide is released from the solution as small bubbles, which causes the solution to become effervescent, or fizzy. A common example is the dissolving of carbon dioxide in water, resulting in carbonated water.
Carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released.
Incorporation into chemical compounds
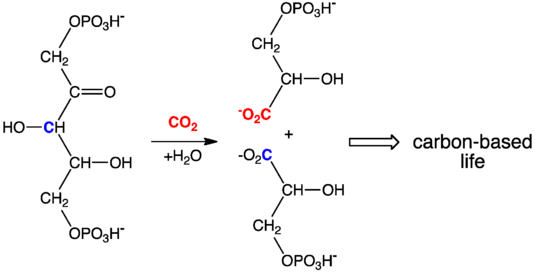
Carbonation of ribulose bisphosphate is the starting point of the incorporation of carbon dioxide into the biosphere.
Carbonation also describes the incorporation of carbon dioxide into chemical compounds. This occurs in the following cases:
- In biochemistry
- Our carbon-based life originates from a carbonation reaction that is most often catalysed by the enzyme RuBisCO. So important is this carbonation process that a significant fraction of leaf mass consists of this carbonating enzyme.[1]
- In reinforced concrete construction
- Also known as neutralisation, it is a chemical reaction between carbon dioxide in the air and calcium hydroxide and hydrated calcium silicate in the concrete.
See also
References
External links
| Look up effervescence in Wiktionary, the free dictionary. |
| Look up carbonation in Wiktionary, the free dictionary. |
- Carbonation and Acidity
- Dissolution of Marble in Hydrochloric Acid Demonstration (instruction and video)
- Robert O'Leary. "ATR Infrared Spectroscopy method for measuring CO2 concentration in Beer" (PDF). Describes in detail the theory and practice of measuring dissolved CO2 content in soft drinks and beer.
This article is issued from Wikipedia - version of the Monday, February 01, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.